Abstract
Diabetic foot lesions remain a major cause of morbidity in patients with renal failure, especially those on dialysis. Foot complications are encountered at a more than twofold frequency in diabetic patients with end-stage renal disease, and the rate of amputations is 6.5–10 times higher in comparison to the general diabetic population. The causal pathways of the diabetic foot in renal failure are multiple and inter-related. Three major pathologies—neuropathy, ischemia, and infection—are the main contributory factors. Increased awareness of this condition and careful clinical examination are indispensable to avoid serious complications. Appropriate management needs to address all contributory factors. Treatment options include revascularization, off-loading to relieve high-pressure areas, and aggressive control of infection. Equally important is the collaboration between health care providers in a multidisciplinary foot care setting. Moreover, patient education on the measures required to achieve both primary and secondary prevention is of great value. Certainly, technical innovations have made considerable progress possible, but there is a need for further improvement to reduce the number of amputations.
INTRODUCTION: DIABETES AND DIABETIC NEPHROPATHY
Diabetes mellitus represents a major health challenge for the 21st century, and its incidence is still rising. Indeed, the total number of diabetic patients has been projected to rise from 171 million in 2000 to 366 million in 2030.Citation[1] The vast majority of these patients have type 2 diabetes, which is showing an unprecedented epidemic,Citation[2] mainly attributable to obesity and a lack of exercise, typical of Western lifestyle.Citation[3] Interestingly, an increased incidence of type 1 diabetes has also been observed, although the reasons are not immediately apparent.Citation[4]
Diabetic nephropathy is a major chronic complication of diabetes with an adverse impact on morbidity and mortality.Citation[5] In the Western countries, it is among the leading causes of end-stage renal disease (ESRD) requiring dialysis.Citation[6],Citation[7] Cases of diabetes-related dialysis are still increasing in these countries.Citation[8] Based on urinary albumin excretion, it is traditionally divided into a first phase of microalbuminuria or incipient nephropathy and a second phase of macroalbuminuria or clinical nephropathy.Citation[6],Citation[7] The latter has been shown to occur in 15–40% of patients with type 1 diabetes and in 5–20% of patients with type 2 diabetes.Citation[6] Patients with diabetic nephropathy have an exceedingly high frequency of further concomitant microvascular diabetic complications, notably neuropathy and retinopathy, but also macrovascular disease.Citation[9] This disease burden ultimately reduces survival.Citation[5–7]
THE DIABETIC FOOT: EPIDEMIOLOGY AND PATHOGENESIS
The diabetic foot also belongs to the most frequent complications of diabetes.Citation[10],Citation[11] More than 5% of diabetic patients have a history of foot ulcers,Citation[10] and the lifetime risk of developing this condition has been estimated at 25%.Citation[12] The pathogenesis of the diabetic foot is complex, and it has a considerable adverse impact on morbidity.Citation[10],Citation[11] Despite the progress so far accomplished in management,Citation[13],Citation[14] the situation continues to be far from promising.Citation[14],Citation[15] Not to be undervalued, every 30 seconds, a lower limb is lost due to diabetes across the world.Citation[15] Not only do amputations have a negative effect on the quality of life, but they also confer a huge financial burden on society.Citation[11] Using 1998 currency, the cost for a lower extremity amputation at the foot level was $43,800, while the cost for a more extensive amputation above the ankle was $66,215.Citation[11] Moreover, mortality in amputees is extremely high.Citation[16]
Three major pathologies—neuropathy, ischemia, and infection—interact by miscellaneous routes, resulting in the diabetic foot (see ).Citation[10],Citation[12],Citation[13] Neuropathy is often considered as the cardinal driving force to ulceration,Citation[10],Citation[12],Citation[17] as it has been implicated in the pathogenesis of foot ulceration in the majority of cases.Citation[17] It renders the feet insensitive to noxious stimuli, such as trauma induced by stepping on a sharp object or, simply, skin injury due to ill-fitting shoes.Citation[10],Citation[12],Citation[17],Citation[18] Injury may be minor, but it goes unrecognized, with resultant progressive tissue damage and superinfection.Citation[10],Citation[12],Citation[17],Citation[19] Furthermore, impaired innervation of foot muscles may lead to muscular atrophy and deformities, notably prominent metatarsal heads, claw toes, or hammer toes.Citation[10],Citation[17–18] Of note, the critical triad comprising neuropathy, minor skin trauma, and deformity appears to account for more than 60% of cases presenting with foot ulcers.Citation[17] A frequently neglected component of neuropathy is diminished sweating, otherwise known as sudomotor impairment.Citation[20],Citation[21] This results in dry skin and callus formation, enabling bacteria to enter the skin through fissures and rendering the foot vulnerable to ulceration.Citation[10],Citation[20] Finally, poor balance and instability, largely ascribable to a loss of proprioception, predispose one to foot trauma and ulceration.Citation[22],Citation[23]
Table 1 Pathogenesis of the diabetic foot and special features in ESRD
Ischemia ensues from peripheral arterial occlusive disease.Citation[10],Citation[12],Citation[13],Citation[24] In diabetes, this manifestation of atherosclerosis occurs more frequently, exhibiting a pattern of diffuse multi-vessel involvement with a predilection for the infrapopliteal arteries.Citation[25],Citation[26] Additionally, diabetic patients have more severe disease and worse outcomes.Citation[25]
Infection is a principal aggravating factor.Citation[27],Citation[28] According to a previous study, more than half of chronic foot ulcers develop infection.Citation[29] More recently, the multicentre EURODIALE study of 1,229 consecutive patients presenting with a new foot ulcer has reported that 58% of the ulcers were infected at presentation, while evidence of infection was present in 82% of patients admitted to hospital.Citation[30] Other important gates of bacterial entry include ingrown toenails, paronychia, and interdigital mycoses.Citation[27],Citation[28] Infection is usually polymicrobial, with a mixed picture of gram-positive cocci, accompanied by gram-negative bacteria and anaerobes.Citation[27],Citation[28] Methicillin-resistant Staphylococcus aureus (MRSA) is becoming a serious threat.Citation[31] The most feared complication is a spread of infection to ligaments, tendons, and bones, which may lead to limb-threatening gangrene even in the absence of macroangiopathy.Citation[27],Citation[28] Indeed, the alarming rapidity at which infection, although appearing deceptively innocuous, may progress to necrosis and sepsis cannot be overestimated. Regrettably, there is often a paucity of symptoms and clinical signs, as tissue response to infection is blunted by ischemia and neuropathy, which may unduly delay treatment.Citation[27]
THE DIABETIC FOOT: PRINCIPLES OF MANAGEMENT
The treatment of diabetic foot ulcers needs to address all three etiologic factors previously described. It has to be efficacious in the correction of ischemia, to compensate for defects due to neuropathy and to tackle the challenge of infection.Citation[10],Citation[13],Citation[24] Any diabetic patient with a non-healing ulcer or clinical suspicion of ischemia must urgently undergo vascular examination to enable the timely revascularization by means of angioplasty or bypass surgery.Citation[32–34] Off-loading the ulcerated area of the foot with casts and cushioning insoles is indispensable to healing in the neuropathic foot.Citation[10],Citation[13] Casting is aided by vigorous surgical debridement, which transforms the chronic wound into an acute one, thereby promoting granulation and healing.Citation[35] Revascularization and debridement will also facilitate the management of infection.Citation[13],Citation[27] The prudent use of antibiotics, choosing broad-spectrum agents initially and subsequently guided by deep swab cultures, is required to treat infection.Citation[13],Citation[27],Citation[28] In addition to these established measures of care, innovative therapies such as bioengineered skin construct and growth factors have been developed and show promise.Citation[36],Citation[37]
THE DIABETIC FOOT IN ESRD
ESRD is associated with a significant increase in the frequency of diabetic foot lesions. This holds true for all foot complications, namely ulceration, infection, gangrene, and amputation, which are encountered at a more than twofold frequency in diabetic patients with ESRD as compared with their non-nephropathic counterparts.Citation[38],Citation[39] Tragically enough, the rate of amputations is 6.5–10 times higher among diabetic patients with ESRD in comparison to the general diabetic population.Citation[39–41] Similarly, the presence of renal disease confers a dismal prognosis in terms of all foot-related outcomes among patients with diabetes.Citation[42–47] Griffiths et al. (1990) found that reduced creatinine clearance increased the risk for foot ulceration.Citation[42] A precipitous rise in the relative risk (RR) for ischemic foot ulceration (RR = 21.580, 95% CI: 4.838–96.251, p < 0.001) conferred by diabetic nephropathy has more recently been confirmed.Citation[43] Major amputation rates are also significantly increased (RR = 2.14; 95%CI: 1.17–3.94, p = 0.0014) in diabetic patients on hemodialysis, who have a reduced survival.Citation[44] Indeed, only 50% of dialysis patients in another study avoided major amputation, despite attempts at revascularization.Citation[45] A significant (p = 0.04) association between ESRD and failure of transmetatarsal amputations to heal has been reported.Citation[46] Interestingly, the increased risk for amputation conferred by renal failure is seen in all ethnic groups in the United States.Citation[47]
Although most pronounced in ESRD, the increased incidence of diabetic foot complications is observed in all stages of diabetic nephropathy. Even as early evidence of nephropathy as microalbuminuria is an independent risk factor for foot ulcer (RR = 8.2, 95%CI: 3.8–18.7, p < 0.0001).Citation[48] However, the incidence of foot complications, naturally, increases with the progression of nephropathy.Citation[49],Citation[50] Both ulcerations and amputations occur in significant association with severity of nephropathy.Citation[49] Patients on dialysis, thus, fare the worst. In the study by Morbach et al. (2001), the rate of amputation was 57% among patients on hemodialysis compared with 25% in those with pre-dialysis renal failure (p = 0.006).Citation[39] In a similar fashion, the risk of poor outcome among longstanding diabetes with critical foot ischemia was increased 8.9 times in those on dialysis.Citation[51]
Moreover, there is a close temporal association between commencement of dialysis and occurrence of foot complications.Citation[52],Citation[53] McGrath and Curran (2000) have reported that the time from initiation of dialysis (either hemodialysis or chronic ambulatory peritoneal dialysis) to amputation was less than 12 months in the majority of patients, with a median of seven months.Citation[52] This is supported by a recent self-controlled case series from Nottingham demonstrating a sharply increased incidence rate of foot ulceration by 3.35 (95% CI: 1.59–7.04) in the first year after initiation of dialysis, followed by an increased rate by 4.56 (95% CI: 2.19–9.5) in the 2nd–5th year.Citation[53] The increased incidence rates of major amputation were 31.98 (95% CI: 2.09–490.3) and 34.01 (95% CI: 1.74–666.2), respectively.Citation[53]
Renal transplant patients represent a special group. Due to the immunosuppressive treatment, they are at increased risk for infection. Accordingly, they may develop foot infections with more resistant bacteria including multi-resistant Staphylococcus, Enterococcus, and Pseudomonas species.Citation[54] These infections are very difficult to treat, often requiring repeated hospital admissions and sometimes leading to life-threatening septicemia.Citation[55] In these patients, amputation rates are particularly high, from 10–30%.Citation[55–57] Moreover, renal transplant recipients frequently have severe and progressive peripheral arterial disease, which is not ameliorated by concomitant pancreas transplantation, despite improved glycemic control.Citation[56],Citation[58]
The causal pathways of the diabetic foot in renal failure are multiple and inter-related. A case has been made both for neuropathyCitation[41] and peripheral arterial disease.Citation[43],Citation[56],Citation[58] Yet foot lesions are, rather, attributable to a combination of both entities.Citation[39],Citation[59],Citation[60] Certainly, diabetic nephropathy, especially ESRD, is accompanied by an increased prevalence of peripheral arterial disease,Citation[5],Citation[6],Citation[60] largely associated with longer diabetes durationCitation[5],Citation[6],Citation[59] but also with a significantly more frequent presence of vascular risk factors.Citation[5],Citation[61] Similarly, neuropathy is particularly common, possibly aggravated by a component of uremic neuropathy.Citation[59] Specifically, the co-existence of neuropathy and peripheral arterial disease has been found to be significantly (p = 0.015) more common in patients on dialysis than in the general diabetic population.Citation[39] An increased propensity to infection, largely explicable due to the effect of uremic toxins and/or poor nutrition, is a further potential contributory factor.Citation[5],Citation[59],Citation[60] Hence, it appears that the three cardinal etiologic factors previously discussed to be of vital importance in the pathogenesis of the diabetic renal foot are aggravated in ESRD (see ).
However, additional factors are also of importance. Uremic autonomic neuropathy may confer further skin fragility, as already mentioned, increasing the likelihood of ulceration.Citation[20],Citation[59],Citation[60] More importantly, postural hypotension, attributable to both disordered vasomotor regulation due to cardiovascular autonomic neuropathy and to post-dialysis volume constriction, increases the risk of trauma.Citation[60] The latter is facilitated by poor vision owing to diabetic retinopathy.Citation[5],Citation[9],Citation[60] Reduced tissue oxygenation due to anemia and tissue edema has also been suggested.Citation[60]
In particular, hemodialysis per se has been proposed as a factor of the utmost importance in the pathogenesis of diabetic foot lesions.Citation[39],Citation[53],Citation[60] This notion would also explain the aforementioned steep rise in amputation rates seen after commencement of dialysis.Citation[39],Citation[52],Citation[53] Lower-extremity ischemia may ensue from systemic hypoxia. The latter is thought to occur due to various factors, including pulmonary microatelectasis, the activation of complement, and a change in the oxygen dissociation curve.Citation[62] Nonetheless, the direct effect of hemodialysis should not lead to an underestimation of perturbations associated with diabetes.Citation[63],Citation[64] Among patients with various kidney diseases requiring dialysis, diabetes significantly increases the risk for amputation by 2.51–6.4.Citation[63],Citation[64] Intradialytic or post-dialysis hypotension may generally elicit a hemodynamic response with reduced peripheral blood flow to preserve central circulation, but these changes are extremely crucial in diabetic patients with distal peripheral vasculopathy and cardiac autonomic neuropathy. Indeed, a trend toward reduced transcutaneous oxygen pressure (TcPO2) on the dorsum of the foot, reflecting poor circulation, for at least four hours after dialysis has been shown in patients with diabetes.Citation[60] Finally, a factor probably overlooked is the major challenge induced by hemodialysis to patients' lifestyle, dramatically changing the emphasis of medical priorities and leading to neglected foot care.Citation[53],Citation[65],Citation[66]
THE DIABETIC FOOT IN RENAL FAILURE: EXAMINATION AND RISK ESTIMATION
Careful clinical evaluation remains the cornerstone of the diagnosis of the diabetic foot.Citation[10],Citation[67],Citation[68] An examination should address all relevant key issues (i.e., peripheral circulation, evidence of neuropathy, factors predisposing to ulceration and presence of infection).Citation[10],Citation[67],Citation[68] Major clinical evaluation methods are presented in .
Table 2 Clinical examination for the prevention and care of the diabetic foot
An elementary sign is presence of palpable peripheral pulses (i.e., pulses on the dorsalis pedis and posterior tibial arteries). Palpable pulses suggest sufficient peripheral circulation, whereas the absence of pulses is a sign of ischemia and represents a recognized risk factor for the development of foot lesions.Citation[10],Citation[67–69] Measurement of the Ankle-Brachial-Index (ABI) by a Doppler apparatus is a simple technique to evaluate the adequacy of blood supply to the limb.Citation[70],Citation[71] An ABI < 0.9 is an index of peripheral arterial disease.Citation[70],Citation[71] The advantages of the ABI include its simplicity, as well as the ability to apply it whenever necessary without having to refer the patient to a specialized vascular laboratory.Citation[70–71] Equally important, it is highly reproducible.Citation[72] Certainly its use in patients with diabetes mellitus has been criticized because arterial medial calcification may cause spuriously elevated ankle pressures, even more so in patients with ESRD.Citation[70],Citation[71],Citation[73],Citation[74] This potential underestimation of peripheral arterial disease may be circumvented by measuring toe pressures, but such measurement requires more sophisticated equipment and trained personnel.Citation[70],Citation[71],Citation[73],Citation[74] Transcutaneous oxygen pressure (TcPO2), Duplex imaging, and angiography represent further modalities used in a more comprehensive evaluation of peripheral circulation, especially prior to revascularization.Citation[33],Citation[70],Citation[71]
Diabetic neuropathy is also diagnosed clinically.Citation[75] Examination includes ankle reflexes, light touch, temperature, pain, and tuning fork sensation.Citation[10],Citation[75] An excellent standardized diagnostic tools is the Neuropathy Disability Score (NDS) comprising ankle reflexes, 128 Hz tuning fork, pin-prick and qualitative temperature sensation at the hallux.Citation[76] Sensation of pressure due to foreign bodies, known as protective sensation, is best evaluated by two standardized and validated tests, the 10 g Semmes Weinstein monofilament and the Vibration Perception Threshold (VPT).Citation[10],Citation[69],Citation[75] The former may easily be applied on the foot (usually on three callus-free plantar sites, such as the first and fifth metatarsal head and the heel) with enough force to cause it to buckle.Citation[69] Reduced monofilament sensation is a strong independent risk factor of foot ulceration.Citation[10],Citation[69],Citation[75] The latter evaluates sensation of an electric stimulus induced by a special device (the Neurothesiometer) on the pulp of the hallux, reduced sensation conferring a substantial risk for development of foot ulcer.Citation[77] More sophisticated, not widely used alternative tests of protective sensation include the tactile circumferential discriminatorCitation[78] and the steel ball-bearing test.Citation[79] Finally, a new simple test measures sweat production by color change of an indicator test applied on the plantar aspect of the foot, thereby additionally evaluating foot skin dryness.Citation[21]
In addition, the clinician should meticulously examine the foot for evidence of increased risk. Heavy callus, prominent metatarsal heads, plantar arch collapse, claw or hammer toes, and other deformities, as well as nail pathology (onychomycosis, ingrown nails, paronychia) and skin changes (blisters, fissures etc) should by no means escape notice.Citation[15],Citation[19],Citation[24],Citation[27],Citation[66–68] In the case of foot ulcer, extent and depth of tissue loss and early signs of infection, such as erythema, discoloration, pus, or friability of granulation tissue, should be carefully examined.Citation[15],Citation[24],Citation[27],Citation[28] Particular attention should be paid not to miss infection between the toes and under the nails.Citation[15],Citation[27] Finally, patients on dialysis that are frequently immobilised should also be carefully examined for the presence of pressure ulcers on the heels.
Foot examination should also include patients' shoes and socks.Citation[15],Citation[24],Citation[66],Citation[68] It is important to identify worn-out or ill-fitting shoes with an extremely high heel or a narrow toe box. High-pressure areas in the shoes should also be examined. Unperceived foreign bodies in the shoes may be the cause of ulceration and should not be overlooked. Too tight socks with inappropriate seams should be identified and discouraged.Citation[15],Citation[24],Citation[66],Citation[68]
In patients presenting with a foot ulcer, it is important to exclude significant ischemia, as this will have a vast significance for treatment.Citation[15],Citation[19],Citation[24] Traditionally, diabetic foot ulcers are classified into two groups: the ischemic or neuroischemic and the merely neuropathic.Citation[13],Citation[15],Citation[19],Citation[24] Signs of ischemia include a cold foot, with absent pulses and a painful ulcer with irregular shape located on the tips of the toes, heel, or margins of the foot.Citation[15],Citation[19],Citation[24] Conversely, the neuropathic foot with intact circulation is usually warm, with strong, at times even bounding, pulses, reduced ankle reflexes, and evidence of sensory loss (pinprick, tuning fork, hot and cold discrimination, monofilament, VPT).Citation[15],Citation[18],Citation[19],Citation[24] The neuropathic ulcer is usually painless, located in areas of high pressure (metatarsal heads, pulp of claw or hammer toes, between the toes, dorsal surface of the toes due to compression by footwear).Citation[15],Citation[18],Citation[19],Citation[24] It originates in areas with preceding callus formation and has a corkscrew appearance, its edges being replete with callus.Citation[15],Citation[18],Citation[19],Citation[24] Other manifestations of neuropathy, such as dry skin or Charcot osteoarthropathy (a form of neuropathic joint destruction, mainly of the midfoot) may be present.Citation[15],Citation[18],Citation[19],Citation[24]
Self-evidently, the clinician should be able to recognize the acutely presenting diabetic foot. This is typically characterized by gangrene, either dry with demarcation or wet with a tendency to spread into adjacent tissue rapidly (see ).Citation[12],Citation[15],Citation[24],Citation[27] Other danger signs not to be missed include redness and swelling accompanied by pain in a previously painless neuropathic foot, indicating a developing abscess; cellulitis; crepitus due to gas in soft tissues caused by infection; and critical ischemia manifesting as a pink, painful, cold, pulseless foot.Citation[12],Citation[15],Citation[24],Citation[27]
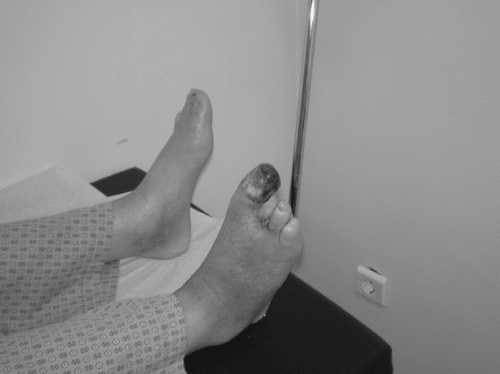
Figure 1. Gangrene of the right hallux in a patient with longstanding diabetes, peripheral arterial disease, and ESRD. The second and third toes have been amputated previously. Deep culture after drainage of the pus in the proximal phalanx of the hallux grew Enterococcus faecalis and Pseudomonas aeruginosa.
THE DIABETIC FOOT IN RENAL FAILURE: PREVENTION AND TREATMENT
Management of the diabetic foot in renal failure follows the same principles as general management of the diabetic foot. It is of foremost importance to diagnose ischemia early and to attempt improving blood flow to the limb.Citation[13],Citation[15],Citation[24],Citation[25],Citation[32],Citation[34],Citation[80] Revascularization may be accomplished either by means of bypass surgery or angioplasty.Citation[13],Citation[32–34] Given that peripheral arterial disease in diabetic patients mostly affects the infra-popliteal arteries,Citation[13],Citation[25],Citation[26] distal bypass grafting is often required, for instance, to the pedal artery.Citation[32–34] If several serial stenoses are present, it may be advisable to correct the most proximal stenosis by angioplasty in combination with distal bypass grafting.Citation[32–34] Regarding revascularization, two fundamental rules should be followed. First, reconstructive surgery should be kept to a minimum required (i.e., no more and no less than needed). Secondly, revascularization should precede local surgery such as debridement or limited amputation, so as to ensure wound healing and infection control.
Improved revascularization in renal patients has enabled increased limb survival.Citation[13],Citation[81] Sakurai et al (1995) performed infra-inguinal arterial reconstruction in 11 diabetic patients with ESRD and reported 42% survival rate at one year, while no graft failure was observed.Citation[82] An 82% primary graft patency at three years was achieved by Treiman et al. (2000) in patients with ESRD and distal peripheral arterial disease.Citation[83] Another group performed infra-inguinal or axillofemoral bypass surgery in diabetic patients with ESRD.Citation[84] Survival was 63% at one year and 45% at two years, while limb salvage rate was 65% at one year. Primary graft patency rates were 64% at one year and 58% at three years.Citation[84] Improved limb salvage and patient survival rates, as well as reduced time to heal, have been accomplished by appropriate revascularization (angioplasty, femoro-popliteal, femoral-distal, popliteal-distal bypass surgery).Citation[85] The department of vascular surgery at Beth Israel Deaconess Medical Center in Boston has reported primary graft patency rates of 84% and 64% at one and three years, respectively.Citation[86] Limb salvage rates were 80% at both one year and three years. Survival rates were 60% and 18% at one and three years, respectively.Citation[86] The importance of arterial reconstruction to re-institute normal blood flow is further enhanced by the observation that critical limb ischemia may be a main cause of death in patients with ESRD.Citation[87]
Revascularization presents a considerable clinical challenge in renal transplant recipients. In a pioneer study, the diabetic foot clinic at King's College Hospital in London showed that a multidisciplinary foot care program including angioplasty and distal bypass succeeded in dramatically reducing gangrene and amputation rates, while healing times for lesions were similar to those reported in diabetic patients without renal transplants.Citation[81] More recently, primary graft patency rates of 78% at one year and 44% at three years, with corresponding limb survival rates of 87% and 78%, have been reported.Citation[88]
When there is adequate circulation, and the major cause leading to foot ulceration is neuropathy, it is of vital importance to relieve high-pressure areas.Citation[10],Citation[13],Citation[15] This is accomplished by bedrest to reduce further weight-bearing on the ulcerated foot and by the immediate application of some form of cast.Citation[10],Citation[13],Citation[15],Citation[89] Casts include removable cast walkers, such as the Aircast walker (a bivalved cast, lined with four air cells and inflated by a hand pump to ensure a close fit) and the Scotch cast boot (a simple removable boot, made of stockinette, felt, and fiberglass tape), and the irremovable total contact cast.Citation[10],Citation[13] The Total Contact cast is an irremovable cast, generally regarded as the “gold standard” in off-loading techniques.Citation[10],Citation[13],Citation[90] It is made of stockinette, low-density foam, elastic plaster, and fiberglass. It has a rocker sole created by layering standard plaster on the bottom of the cast from the heel to an area just proximal to the metatarsal heads, facilitating ambulation.Citation[91] Recent evidence suggests that good results may also be achieved by use of the “instant Total Contact Cast,” a removable cast walker rendered irremovable by wrapping in a layer of cohesive or plaster bandage.Citation[89] In case casting facilities are not available, alternative off-loading modalities include temporary ready-made shoes with a cushioning insole, special half shoes for forefoot ulceration, or other orthotic devices.Citation[10],Citation[13],Citation[90] Additionally, crutches, wheelchairs, and Zimmer frames aid in the reduction of weight-bearing.Citation[10],Citation[13]
It is now accepted that the neuropathic ulcer needs frequent surgical debridement by a scalpel.Citation[13],Citation[19],Citation[92] The aim of debridement is manifold. It reduces plantar pressure by callus removal and removes necrotic tissue, bacterial burden, and senescent cells, thereby promoting drainage of exudate, infection control, and the development of healthy granulation tissue.Citation[13],Citation[19],Citation[92] Moreover, it reveals the true dimensions of the ulcer and enables a deep swab or tissue culture to be taken.Citation[13],Citation[19] Non-surgical modes of debridement include autolytic, enzymatic, mechanical (high-pressure irrigation, whirlpool hydrotherapy and ultrasound), and biological (use of sterile maggots) debridement.Citation[93],Citation[94]
Management of infection is the other critical issue. Diagnosis should remain clinical and be accompanied by a high level of suspicion.Citation[13],Citation[27],Citation[28] The clinician should prescribe antibiotics when there is any clinical evidence of infection imposed on a foot ulcer, including minor signs, such as erythema, discolouration or friable granulation tissue.Citation[27] It is impossible to predict the species of bacteria from the clinical presentation.Citation[13],Citation[24],Citation[27] Hence, one should initially prescribe broad-spectrum antibiotics and immediately send tissue or a deep-wound swab for culture.Citation[13],Citation[24],Citation[27] A reliable rule is that the initial regimen should by all means cover gram-positive cocci (including Staphylococcus), while necrosis increases the possibility of gram-negative bacteria and/or anaerobes.Citation[27] The extension of infection to the tendons or bones, cellulitis, gangrene, and constitutional symptoms (fever, malaise, nausea, lethargy, metabolic derangement) should prompt immediate hospitalization for intravenous antibiotic administration and patient monitoring.Citation[24],Citation[27],Citation[28]
Diabetic gangrene is a special condition requiring urgent hospital admission and treatment.Citation[24],Citation[95] Management should differ according to the underlying pathology and the stage of necrotic process. The need to diagnose ischemia and improve blood flow cannot be overemphasized.Citation[13],Citation[24],Citation[27],Citation[28] In the absence of ischemia, surgical debridement, removal of necrotic tissue, and aggressive broad-spectrum antibiotic treatment are indicated.Citation[27],Citation[28] Aggressive debridement should be frequently repeated in neuropathic patients. Should tendons and bones be affected, surgery needs to be considered. However, this is best done after gaining an initial control of infection.Citation[27],Citation[28] Ischemic gangrenous toes should be kept dry, the surrounding skin should be cleaned, and the adjacent toes should be protected in order to prevent spread of necrosis.Citation[27] If possible, one should anticipate spontaneous demarcation or even auto-amputation.Citation[27],Citation[28]
Additional treatment options in the diabetic renal foot include VAC pump therapy and a local application of agents promoting wound closure.Citation[96–98] Vacuum-assisted closure (VAC) is an established technique promoting healing via a pump creating negative pressure within the wound.Citation[27] It has successfully been applied to diabetic foot ulcers, accomplishing faster wound closure and, in most patients, eliminating the need for further surgery.Citation[27] Experience in diabetic patients with renal failure is limited but encouraging.Citation[27],Citation[96] Agents that have shown promise in improving the healing of the diabetic renal foot include phenytoinCitation[97] and platelet-derived growth factor (PDGF),Citation[98] but experience is only limited to case reports.
Obviously, the diabetic foot requires a comprehensive approach to investigate and address all relevant issues.Citation[13–15] This is the case even more so for the diabetic foot in renal failure.Citation[81] There is evidence that the incidence of amputations may be substantially reduced by the provision of an integrated program of care for renal patients, especially those on dialysis.Citation[99–101] Alternately, a program of education and surveillance by a podiatrist may be very effective.Citation[102] It appears that the introduction of such services should become an urgent priority for all dialysis units. It should also be stressed that acquaintance with the diabetic foot in terms of simple rules should be widely available to all health care providers involved in the management of diabetic patients.Citation[103] In particular, dialysis nurses should be in an optimal position to make a positive impact on amputation rates by routine foot screening, education tailored to the patients' needs and understanding, and prevention of ulcer-initiating events.Citation[68],Citation[80],Citation[104]
Prevention of the diabetic foot has made some progress, but it is still inadequate.Citation[14] Adequate patient education and motivation, as well as regular foot examination to correct pre-ulcerous conditions, are of great value and should be continuously pursued.Citation[15],Citation[105] Suitable footwear with properly fitted in-soles and enough space between the toes is mandatory.Citation[15],Citation[106] Patients with deformities or a history of foot ulcer need special bespoke shoes Citation[15]. It is now becoming apparent that zealous proactive care may result in fewer amputations and a higher quality of life.Citation[107] This initiative deserves to be applied to the particularly vulnerable population of ESRD patients.
CONCLUSIONS
Diabetic foot lesions remain a major cause of morbidity in patients with renal failure, especially those on dialysis. Indeed, foot complications are encountered at a more than twofold frequency in diabetic patients with ESRD, and the rate of amputations is 6.5–10 times higher in comparison to the general diabetic population. Increased awareness of this condition and meticulous clinical examination are indispensable to avoiding serious complications. Appropriate management needs to address all three contributory factors, notably ischemia, neuropathy, and infection. Treatment options include revascularization, off-loading to relieve high-pressure areas, and aggressive control of infection. Equally important is the collaboration between health care providers in a multidisciplinary foot care setting. Moreover, patient education on the measures required to achieve both primary and secondary prevention is of great value. Certainly, technical innovations have enabled considerable progress to be accomplished, but there is a need for further improvement to reduce the number of amputations.
REFERENCES
- Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004; 27: 1047–1053
- Coliaguri S, Borch-Johnsen K, Glümer C, et al. There really is an epidemic of type 2 diabetes. Diabetologia. 2005; 48: 1459–1463
- Zimmet P. Globalization, coca-colonization and the chronic disease epidemic: can the Doomsday scenario be averted?. J Intern Med. 2000; 247: 301–310
- Gale EAM. Spring harvest? Reflections on the rise of type 1 diabetes. Diabetologia. 2005; 48: 2445–2450
- Bloomgarden ZT. Diabetic nephropathy. Diabetes Care. 2005; 28: 745–751
- Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005; 28: 164–176
- Locatelli F, Pozzoni P, Del Vecchio L. Renal replacement therapy in patients with diabetes and end-stage renal disease. J Am Soc Nephrol. 2004; 15(Suppl. 1)S25–S29
- Lok CE, Oliver MJ, Rothwell DM, Hux JE. The growing volume of diabetes-related dialysis: a population based study. Nephrol Dial Transplant. 2004; 19: 3098–3103
- Bloomgarden ZT. Diabetes complications. Diabetes Care. 2004; 27: 1506–1514
- Boulton AJM. The diabetic foot: from art to science. The 18th Camillo Golgi lecture. Diabetologia. 2004; 47: 1343–1353
- Boulton AJM, Vileikyte L, Ragnarson-Tennvall G, et al. The global burden of diabetic foot disease. Lancet. 2005; 366: 1719–1724
- Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005; 293: 217–228
- Edmonds M. The diabetic foot, 2003. Diabetes Metab Res Rev. 2004; 20(Suppl. 1)S9–S12
- Papanas N, Maltezos E, Edmonds M. St. Vincent declaration after 15 years or who cleft the devil's foot?. Vasa. 2006; 35: 3–4
- Time to Act, K Bakker, AVM Foster, WH van Houtoum, P Riley. International Diabetes Federation and International Working Group of the Diabetic Foot, AmsterdamThe Netherlands 2005
- Tentolouris N, Al-Sabbagh S, Walker MG, et al. Mortality in diabetic and nondiabetic patients after amputations performed from 1990 to 1995: a five-year follow-up study. Diabetes Care. 2004; 27: 1598–1604
- Reiber GE, Vileikyte L, Boyko EJ, et al. Causal pathways for incident lower-extremity ulcers in patients with diabetes from two settings. Diabetes Care. 1999; 22: 157–162
- Boulton AJ, Kirsner RS, Vileikyte L. Clinical practice. Neuropathic diabetic foot ulcers. N Engl J Med. 2004; 351: 48–55
- Edmonds ME, Foster AVM, Sanders LJ. Stage 3: the ulcerated foot. A Practical Manual of Diabetic Footcare, ME Edmonds, AVM Foster, LJ Sanders. Blackwell, Oxford 2004; 62–101
- Low PA. Sudomotor function. Textbook of Diabetic Neuropathy, FA Gries, PA Low, NE Cameron, D Ziegler. Georg Thieme Verlag, Stuttgart 2003; 274–278
- Papanas N, Papatheodorou K, Christakidis D, et al. Evaluation of a new indicator test for sudomotor function (Neuropad) in the diagnosis of peripheral neuropathy in type 2 diabetic patients. Exp Clin Endocrinol Diabetes. 2005; 113: 195–198
- Katoulis EC, Ebdon-Parry M, Hollis S, et al. Postural instability in diabetic neuropathic patients at risk of foot ulceration. Diabet Med. 1997; 14: 296–300
- Katoulis EC, Ebdon-Parry M, Lanshammar H, Vileikyte L, Kulkarni J, Boulton AJ. Gait abnormalities in diabetic neuropathy. Diabetes Care. 1997; 20: 1904–1907
- Watkins PJ. The diabetic foot. BMJ. 2003; 326: 977–979
- Jude EB, Oyibo SO, Chalmers N, Boulton AJ. Peripheral arterial disease in diabetic and nondiabetic patients: a comparison of severity and outcome. Diabetes Care. 2001; 24: 1433–1437
- Kallio M, Forsblom C, Groop PH, Groop L, Lepantalo M. Development of new peripheral arterial occlusive disease in patients with type 2 diabetes during a mean follow-up of 11 years. Diabetes Care. 2003; 26: 1241–1245
- Edmonds ME, Foster AVM, Sanders LJ. Stage 4: the infected foot. A Practical Manual of Diabetic Footcare, ME Edmonds, AVM Foster, LJ Sanders. Blackwell, Oxford 2004; 102–140
- Lipsky BA, Berendt AR, Deery HG, et al. Diagnosis and treatment of diabetic foot infections. Plast Reconstr Surg. 2006; 117(7 Suppl)212S–238S
- Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care. 1990; 13: 513–521
- Prompers L, Huijberts M, Apelqvist J, et al. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia. 2007; 50: 18–25
- Tentolouris N, Jude EB, Smirnof I, Knowles EA, Boulton AJ. Methicillin-resistant Staphylococcus aureus: an increasing problem in a diabetic foot clinic. Diabet Med. 1999; 16: 767–771
- Lepantalo M, Biancari F, Tukiainen E. Never amputate without consultation of a vascular surgeon. Diabetes Metab Res Rev. 2000; 16(Suppl. 1)S27–S32
- Andros G. Diagnostic and therapeutic arterial interventions in the ulcerated diabetic foot. Diabetes Metab Res Rev. 2000; 20(Suppl. 1)S29–S33
- Sumpio BF, Lee T, Blume PA. Vascular evaluation and arterial reconstruction of the diabetic foot. Clin Podiatr Med Surg. 2003; 20: 689–708
- Steed DL, Donohoe D, Webster MW, et al. Effect of extensive debridement and treatment on the healing of diabetic foot ulcers. Diabetic Ulcer Study Group. J Am Coll Surg. 1996; 183: 61–64
- Shen JT, Falanga V. Innovative therapies in wound healing. J Cutan Med Surg. 2003; 7: 217–224
- Petrova N, Edmonds M. Emerging drugs for diabetic foot ulcers. Expert Opin Emerg Drugs. 2006; 11: 709–724
- Hill MN, Feldman HI, Hilton SC, Holechek MJ, Ylitalo M, Benedict GW. Risk of foot complications in long-term diabetic patients with and without ESRD: a preliminary study. ANNA J. 1996; 23: 381–386
- Morbach S, Quante C, Ochs HR, Gaschler F, Pallast JM, Knevels U. Increased risk of lower-extremity amputation among Caucasian diabetic patients on dialysis. Diabetes Care. 2001; 24: 1689–1690
- Eggers PW, Gohdes D, Pugh J. Nontraumatic lower extremity amputations in the Medicare end-stage renal disease population. Kidney Int. 1999; 56: 1524–1533
- Deery HG, II, Sangeorzan JA. Saving the diabetic foot with special reference to the patient with chronic renal failure. Infect Dis Clin North Am. 2001; 15: 953–981
- Griffiths GD, Wieman TJ. The influence of renal function on diabetic foot ulceration. Arch Surg. 1990; 125: 1567–1569
- Yasuhara H, Naka S, Yanagie H, Nagawa H. Influence of diabetes on persistent nonhealing ischemic foot ulcer in end-stage renal disease. World J Surg. 2002; 26: 1360–1364
- Miyajima S, Shirai A, Yamamoto S, Okada N, Matsushita T. Risk factors for major limb amputations in diabetic foot gangrene patients. Diabetes Res Clin Pract. 2006; 71: 272–279
- Stone PA, Back MR, Armstrong PA, et al. Midfoot amputations expand limb salvage rates for diabetic foot infections. Ann Vasc Surg. 2005; 19: 805–811
- Pollard J, Hamilton GA, Rush SM, Ford LA. Mortality and morbidity after transmetatarsal amputation: retrospective review of 101 cases. J Foot Ankle Surg. 2006; 45: 91–97
- Young BA, Maynard C, Reiber G, Boyko EJ. Effects of ethnicity and nephropathy on lower-extremity amputation risk among diabetic veterans. Diabetes Care. 2003; 26: 495–501
- Guerrero-Romero F, Rodriguez-Moran M. Relationship of microalbuminuria with the diabetic foot ulcers in type II diabetes. J Diabetes Complications. 1998; 12: 193–196
- Schleiffer T, Holken H, Brass H. Morbidity in 565 type 2 diabetic patients according to stage of nephropathy. J Diabetes Complications. 1998; 12: 103–109
- Alebiosu CO, Odusan O, Jaiyesimi A. Morbidity in relation to stage of diabetic nephropathy in type-2 diabetic patients. J Natl Med Assoc. 2003; 95: 1042–1047
- Volaco A, Chantelau E, Richter B, Luther B. Outcome of critical foot ischaemia in longstanding diabetic patients: a retrospective cohort study in a specialised tertiary care centre. Vasa. 2004; 33: 36–41
- McGrath NM, Curran BA. Recent commencement of dialysis is a risk factor for lower-extremity amputation in a high-risk diabetic population. Diabetes Care. 2000; 23: 432–433
- Game FL, Chipchase SY, Hubbard R, Burden RP, Jeffcoate WJ. Temporal association between the incidence of foot ulceration and the start of dialysis in diabetes mellitus. Nephrol Dial Transplant. 2006; 21: 3207–3210
- Fejfarova V, Jirkovska A, Petkov V, Boucek P, Skibova J. Comparison of microbial findings and resistance to antibiotics between transplant patients, patients on hemodialysis, and other patients with the diabetic foot. J Diabetes Complications. 2004; 18: 108–112
- George RK, Verma AK, Agarwal A, Mishra SK. An audit of foot infections in patients with diabetes mellitus following renal transplantation. Int J Lower Extrem Wounds. 2004; 3: 157–160
- Kalker AJ, Pirsch JD, Heisey D, et al. Foot problems in the diabetic transplant recipient. Clin Transplant. 1996; 10: 503–510
- Nyberg G, Hartso M, Mjornstedt L, Norden G. Type 2 diabetic patients with nephropathy in a Skandinavian kidney-transplant population. Scand J Urol Nephrol. 1996; 30: 317–322
- Morrissey PE, Shaffer D, Monaco AP, Conway P, Madras PN. Peripheral vascular disease after kidney-pancreas transplantation in diabetic patients with end-stage renal disease. Arch Surg. 1997; 132: 358–361
- Hinchliffe RJ, Jeffcoate WJ, Game FL. Diabetes, established renal failure and the risk to the lower limb. Pract Diab Int. 2006; 23: 28–32
- Hinchliffe RJ, Kirk B, Bhattacharjee D, Roe S, Jeffcoate W, Game F. The effect of haemodialysis on transcutaneous oxygen tension in patients with diabetes—a pilot study. Nephrol Dial Transplant. 2006; 21: 1981–1983
- Kohlhagen J, Kelly J. Prevalence of vascular risk factors and vascular disease in predialysis chronic renal failure. Nephrology (Carlton). 2003; 8: 274–279
- Dhakal MP, Kallay MC, Talley TE. Hemodialysis associated hypoxia extends into the post-dialysis period. Int J Artif Organs. 1997; 20: 204–207
- Jaar BG, Astor BC, Berns JS, Powe NR. Predictors of amputation and survival following lower extremity revascularization in hemodialysis patients. Kid Inter. 2004; 65: 613–620
- Speckman RA, Frankenfield DR, Roman SH, et al. Diabetes is the strongest risk factor for lower-extremity amputation in new hemodialysis patients. Diabetes Care. 2004; 27: 2198–2203
- Richbourg MJ. Preventing amputations in patients with end stage renal disease: whatever happened to foot care?. ANNA J. 1998; 25: 13–20
- Lawrence A. Foot care education in renal patients with diabetes. EDTNA ERCA J. 2004; 30: 153–156
- International Working Group of the Diabetic Foot. International Consensus on the Diabetic Foot. The International Working Group on the Diabetic Foot, AmsterdamThe Netherlands 1999
- Fletcher J. Full nursing assessment of patients at risk of diabetic foot ulcers. Br J Nurs. 2006; 15: S18–S21
- Abbott CA, Carrington AL, Ashe H, et al. The North-West Diabetes Foot Care Study: incidence of, and risk factors for, new diabetic foot ulceration in a community-based patient cohort. Diabet Med. 2002; 19: 377–384
- Dormandy JA, Rutherford RB. Management of Peripheral Arterial Disease, Trans-Atlantic Inter-Society Consensus (TASC). European Journal of Vascular and Endovascular Surgery. 2000; 19(6 Suppl. A)S1–S250
- Hiatt WR. Medical treatment of peripheral arterial disease and claudication. N Engl J Med. 2001; 344: 1608–1621
- Stoffers J, Kaiser V, Kester A, Schouten H, Knottnerus A. Peripheral arterial occlusive disease in general practice: the reproducibility of the Ankle-Arm Systolic Pressure Ratio. Scand J Prim Health Care. 1991; 9: 109–114
- Brooks P, Dean R, Patel S, Wu B, Molyneaux L, Yue DK. TBI or not TBI: that is the question. Is it better to measure toe pressure than ankle pressure in diabetic patients?. Diabetic Medicine. 2001; 18: 528–532
- Williams DT, Harding KG, Price P. An evaluation of the efficacy of methods used in screening for lower-limb arterial disease in diabetes. Diabetes Care. 2005; 28: 2206–2210
- Boulton AJM, Vinik AI, Arezzo JC, et al. Diabetic neuropathies. A statement by the American Diabetes Association. Diabetes Care. 2005; 28: 956–962
- Young M, Boulton AJM, McLeod AF, Williams DRR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia. 1993; 36: 150–154
- Young MJ, Breddy JL, Veves A, Boulton AJM. The prediction of diabetic neuropathic foot ulceration using vibration perception thresholds. Diabetes Care. 1994; 17: 557–560
- Vileikyte L, Hutchings G, Hollis S, Boulton AJM. The tactile circumferential discriminator: a new, simple screening device to identify patients at risk of foot ulceration. Diabetes Care. 1997; 20: 623–626
- Papanas N, Gries A, Maltezos E, Zick R. The steel ball-bearing test: a new test for evaluating protective sensation in the diabetic foot. Diabetologia. 2006; 49: 739–743
- Broersma A. Preventing amputations in patients with diabetes and chronic kidney disease. Nephrol Nurs J. 2004; 31: 53–62
- Foster AV, Snowden S, Grenfell A, Watkins PJ, Edmonds ME. Reduction of gangrene and amputations in diabetic renal transplant patients: the role of a special foot clinic. Diabet Med. 1995; 12: 632–635
- Sakurai T, Kobayashi M, Harasawa H, Itoh A, Yamazaki C, Masuko K. Infrainguinal arterial reconstruction in end-stage renal disease. Cardiovasc Surg. 1995; 3: 46–49
- Treiman GS, Lawrence PF, Rockwell WB. Autogenous arterial bypass grafts: durable patency and limb salvage in patients with inframalleolar occlusive disease and end-stage renal disease. J Vasc Surg. 2000; 32: 13–22
- Georgopoulos S, Filis K, Vourliotakis G, et al. Lower extremity bypass procedures in diabetic patients with end-stage renal disease: is it worthwhile?. Nephron Clin Pract. 2005; 99: c37–c41
- Attinger CE, Ducic I, Neville RF, Abbruzzese MR, Gomes M, Sidawy AN. The relative roles of aggressive wound care versus revascularization in salvage of the threatened lower extremity in the renal failure diabetic patient. Plast Reconstr Surg. 2002; 109: 1281–1290
- Ramdev P, Rayan SS, Sheahan M, et al. A decade experience with infrainguinal revascularization in a dialysis-dependent patient population. J Vasc Surg. 2002; 36: 969–974
- Koch M, Trapp R, Kulas W, Grabensee B. Critical limb ischaemia as a main cause of death in patients with end-stage renal disease: a single-centre study. Nephrol Dial Transplant. 2004; 19: 2547–2552
- McArthur CS, Sheahan MG, Pomposelli FB, Jr, et al. Infrainguinal revascularization after renal transplantation. J Vasc Surg. 2003; 37: 1181–1185
- Armstrong DG, Lavery LA, Wu S, Boulton AJ. Evaluation of removable and irremovable cast walkers in the healing of diabetic foot wounds: a randomized controlled trial. Diabetes Care. 2005; 28: 551–554
- Boulton AJ. Pressure and the diabetic foot: clinical science and offloading techniques. Am J Surg. 2004; 187(5A)17S–24S
- Myerson M, Papa J, Eaton K, Wilson K. The total-contact cast for management of neuropathic plantar ulceration of the foot. J Bone Joint Surg Am. 1992; 74: 261–269
- Steed DL, Donohoe D, Webster MW, Lindsley L. Effect of extensive debridement and treatment on the healing of diabetic foot ulcers. Diabetic Ulcer Study Group. J Am Coll Surg. 1996; 183: 61–64
- Ennis WJ, Meneses P. Factors impeding wound healing. Wound healing alternatives in management, LC Kloth, JM McCulloch. FA Davis, Philadelphia 2002; 68–96
- Jeffcoate W, Price P, Harding KG. Wound healing and treatments for people with diabetic foot ulcers. Diabetes Metab Res Rev. 2004; 20(Suppl 1)S78–S89
- Pinzur MS, Slovenkai MP, Trepman E, Shields NN. Diabetes Committee of American Orthopaedic Foot and Ankle Society. Guidelines for diabetic foot care: recommendations endorsed by the Diabetes Committee of the American Orthopaedic Foot and Ankle Society. Foot Ankle Int. 2005; 26: 113–119
- Mendonca DA, Cosker T, Makwana NK. Vacuum-assisted closure to aid wound healing in foot and ankle surgery. Foot Ankle Int. 2005; 26: 761–766
- Spaia S, Eleftheriadis T, Pazarloglou M, et al. Phenytoin efficacy in treating the diabetic foot ulcer of a haemodialysis patient. Nephrol Dial Transplant. 2004; 19: 753
- Tarroni G, Tessarin C, De Silvestro L, et al. Local therapy with platelet-derived growth factors for chronic diabetic ulcers in haemodialysis patients. G Ital Nefrol. 2002; 19: 630–633
- Rith-Najarian S, Gohdes D. Preventing amputations among patients with diabetes on dialysis. Diabetes Care. 2000; 23: 1445–1446
- McGrath NM, Curran BA. Response to Rith-Najarian and Gohdes. Diabetes Care. 2000; 23: 1445–1446
- McMurray SD, Johnson G, Davis S, McDougall K. Diabetes education and care management significantly improve patient outcomes in the dialysis unit. Am J Kidney Dis. 2002; 40: 566–575
- Lipscombe J, Jassal SV, Bailey S, Bargman JM, Vas S, Oreopoulos DG. Chiropody may prevent amputations in diabetic patients on peritoneal dialysis. Perit Dial Int. 2003; 23: 255–259
- Papanas N, Edmonds A, Maltezos E, Edmonds M. The Ten Commandments of the diabetic foot. BMJ. 2005; 331: 1497
- Neil JA, Knuckey CJ, Tanenberg RJ. Prevention of foot ulcers in patients with diabetes and end-stage renal disease. Nephrol Nurs J. 2003; 30: 39–43
- Valk GD, Kriegsman DM, Assendelft WJ. Patient education for preventing diabetic foot ulceration. Cochrane Database Syst Rev. 2005; 1: CD001488
- Cavanagh PR. Therapeutic footwear for people with diabetes. Diabetes Metab Res Rev. 2004; 20(Suppl. 1)S51–S55
- Armstrong DG. Is diabetic foot care efficacious or cost effective?. Ostomy Wound Manage. 2001; 47: 28–32