Abstract
To explore the renoprotective and anti-inflammatory effects of pravastatin, we analyzed the changes in renal function and urinary monocyte chemoattractant protein-1 (MCP-1) level as a renal tubulointerstitial inflammatory biomarker and serum MCP-1 level as a systemic inflammatory biomarker following the introduction of treatment with 10 mg/day of pravastatin in 10 hyperlipidemic type 2 diabetic patients with normoalbuminuria. Twelve months of the pravastatin treatment did not affect urinary levels of albumin, transferrin, N-acetylglucosaminidase, or MCP-1 in the hyperlipidemic diabetic patients, whereas the treatment significantly reduced serum levels of MCP-1 in the patients. The pravastatin treatment effectively lowered low-density lipoprotein cholesterol (LDL-C) levels in the hyperlipidemic diabetic patients to levels nearly to those in 11 non-hyperlipidemic type 2 diabetic patients with normoalbuminuria. Interestingly, serum MCP-1 levels were significantly lower in the hyperlipidemic patients treated with pravastatin than in the non-hyperlipidemic patients. No significant correlation was observed between serum LDL-C and MCP-1 levels in all the data in the hyperlipidemic patients before and after the pravastatin treatment and in the non-hyperlipidemic patients. These results collectively indicate that pravastatin may ameliorate systemic vascular inflammation rather than local renal inflammation in hyperlipidemic type 2 diabetic patients with normoalbuminuria, independent of its cholesterol-lowering effects.
INTRODUCTION
It is well established that low-density lipoprotein cholesterol (LDL-C) is an important risk factor for atherosclerosis, atherothrombosis, and subsequent cardiovascular disease (CVD) in type 2 diabetic patients.Citation[1],Citation[2] Statins are lipid-lowering agents that principally reduce LDL-C by inhibiting the activity of the enzyme 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductaseCitation[3],Citation[4] and are widely used in type 2 diabetic patients. Recent randomized placebo-controlled trials, such as the Heart Protection Study (HPS)Citation[5] and the Collaborative Atorvastatin Diabetes Study (CARDS),Citation[6] have confirmed the long-term benefits of reducing LDL-C levels with intensive statin therapy on the incidence of major cardiovascular events in type 2 diabetic patients.
Notably, it has been suggested that statins may provide systemic anti-inflammatory effects beyond the known LDL-C-lowering effects, which are referred to as pleiotropic effects.Citation[3],Citation[4],Citation[7] This possibility is supported by the results from several clinical studies. A prospective study reported that treatment with pravastatin (40 mg/day) for 24 weeks significantly reduced plasma levels of a systemic inflammatory biomarker C-reactive protein (CRP) in hyperlipidemic patients in a largely LDL-C-independent manner.Citation[8] Furthermore, a crossover trial indicated that treatment with either simvastatin (20 mg/day) or pravastatin (40 mg/day) or atorvastatin (10 mg/day) for six weeks significantly decreased plasma levels of high-sensitivity CRP (hsCRP) in hyperlipidemic patients with no relationship between reductions in hsCRP and LDL-C levels.Citation[9]
Thus, statins are known to reduce the risk of CVD in type 2 diabetic patients through their LDL-C-lowering and perhaps anti-inflammatory effects. However, it remains to be established whether statins provide renoprotective effects in type 2 diabetic patients. In particular, it seems clinically important to determine whether aggressive statin therapy before the development of diabetic nephropathy confers the benefits on the preservation of renal function and mitigation of inflammation in the kidney.
Monocyte chemoattractant protein-1 (MCP-1) is a chemokine that plays a pivotal role in inflammatory responses and atherosclerotic lesion formation.Citation[10],Citation[11] The primary function of MCP-1 is the recruitment of monocytes/macrophages into inflammatory sites.Citation[12] Through the chemotactic effect, MCP-1 mediates the initial step in atherogenesis.Citation[10],Citation[11] In the kidney, MCP-1 is produced by mesangial cellsCitation[13] and tubular epithelial cellsCitation[14] and is considered to mediate renal tubulointerstitial inflammation, tubular atrophy, and interstitial fibrosis.Citation[15],Citation[16] While the circulating level of MCP-1 reflects the degree of systemic vascular inflammation, we have recently demonstrated that urinary excretion level of MCP-1 represents the degree of renal tubulointerstitial inflammation and tubular damage in proteinuric renal diseases, such as diabetic nephropathyCitation[17] and IgA nephropathy.Citation[18]
To explore the renoprotective and anti-inflammatory effects of statins in type 2 diabetic patients before the development of nephropathy, we analyzed the changes in clinical markers of renal disease and urinary MCP-1 level as a renal tubulointerstitial inflammatory biomarker, as well as serum MCP-1 level as a systemic inflammatory biomarker, following the introduction of pravastatin treatment in hyperlipidemic type 2 diabetic patients with normoalbuminuria.
MATERIALS AND METHODS
Patients and Study Protocol
Ten type 2 diabetic patients with hyperlipidemia (LDL-C ≥120 mg/dL) and normoalbuminuria (urinary albumin to creatinine ratio [ACR] <30 mg/g creatinine) were recruited for this study. In addition, 11 age-matched type 2 diabetic patients without hyperlipidemia (LDL-C <120 mg/dL and triglycerides <150 mg/dL) and with normoalbuminuria were also examined to obtain clinical characteristics and parameters at baseline. All patients had never been treated with lipid-lowering agents when enrolled. They also had no history of cardiovascular or renal diseases, cancer, or genetic familial hyperlipidemias. The baseline characteristics of these patients are summarized in .
Table 1 Baseline characteristics of hyperlipidemic and non-hyperlipidemic type 2 diabetic patients with normoalbuminuria
After the 10 diabetic patients with hyperlipidemia started pravastatin treatment (10 mg/day), they visited Akita University Hospital to receive a physical examination and routine laboratory tests at 28-day intervals. Fasting serum and overnight urine samples were collected at baseline and at 6 and 12 months following the introduction of pravastatin treatment, and stored at −85°C before testing. Using these samples, we measured serum and urinary MCP-1, serum hsCRP, and urinary albumin, transferrin, and N-acetylglucosaminidase (NAG) levels, and analyzed changes in the markers of inflammation and renal disease during the pravastatin treatment.
This study was approved by the Institutional Review Board of the Akita University School of Medicine, Akita, Japan. Informed consent was obtained from all study participants.
Laboratory Assays
Serum and urinary MCP-1 levels were measured using a commercially available ELISA kit (R & D Systems, Minneapolis, Minnesota, USA). Serum hsCRP levels were determined by latex agglutination (CRP-Latex II; Denka-Seiken, Tokyo, Japan). Urinary NAG levels were determined by the m-cresol purple method (Shionogi, Osaka, Japan). Urinary albumin and transferrin levels were measured by radioimmunoassay (RIA) as previously described.Citation[19] Urinary excretion levels of MCP-1, NAG, albumin, and transferrin were expressed as the ratio of urinary concentrations of MCP-1, NAG, albumin, and transferrin to grams of urinary creatinine.
Statistical Analysis
Statistical analyses were performed with GraphPad Prism software system (GraphPad, San Diego, California, USA). Data are presented as means ± SE. Paired t test was used to determine the effects of pravastatin treatment on clinical parameters in the same patient. Comparisons of data between two patient groups of hyperlipidemia and non-hyperlipidemia were performed using unpaired t test. Correlation was calculated by Pearson's correlation analysis. A p value of less than 0.05 was considered statistically significant.
RESULTS
summarizes baseline characteristics of hyperlipidemic and non-hyperlipidemic type 2 diabetic patients with normoalbuminuria. There were no significant differences in age, body mass index, and diabetes duration between the two patient groups of hyperlipidemia and non-hyperlipidemia. As shown in , the two patient groups of hyperlipidemia and non-hyperlipidemia were well matched regarding fasting plasma glucose, HbA1c, systolic blood pressure (BP), diastolic BP, BUN, serum creatinine, and urinary albumin at baseline. On the other hand, the levels of LDL-C and triglycerides were significantly lower in the non-hyperlipidemic group than in the hyperlipidemic group at baseline.
Table 2 Changes in clinical parameters during pravastatin treatment in hyperlipidemic type 2 diabetic patients with normoalbuminuria and baseline data in non-hyperlipidemic type 2 diabetic patients with normoalbuminuria
also shows the changes in clinical parameters during pravastatin treatment in the hyperlipidemic group. Fasting plasma glucose, HbA1c, systolic BP, diastolic BP, BUN, and serum creatinine were not changed during the treatment. Expectedly, the levels of LDL-C and remnant-like particle cholesterol (RLP-C) were significantly reduced at 6 and 12 months following the introduction of the treatment compared to the baseline levels. The levels of triglycerides were significantly decreased after 12 months of the treatment relative to the baseline levels. The levels of high-density lipoprotein cholesterol (HDL-C) and lipoprotein (a) (Lp[a]) were not significantly altered during the treatment. While the pravastatin treatment obviously improved hyperlipidemia, the treatment did not affect the levels of urinary albumin and transferrin as markers of renal glomerular damage and also urinary NAG as a marker of renal tubular injury. The levels of serum hsCRP, a systemic inflammatory biomarker, were significantly lowered at 6 and 12 months following the introduction of the pravastatin treatment compared to the baseline levels.
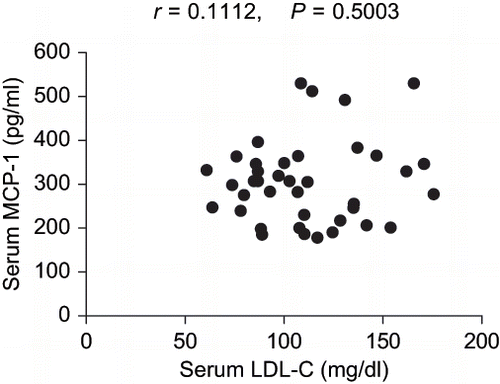
indicates serum and urinary MCP-1 levels in the two patient groups of hyperlipidemia and non-hyperlipidemia. In the hyperlipidemic group, serum MCP-1 levels were significantly decreased at 6 and 12 months following the introduction of the pravastatin treatment compared to the baseline levels (274 ± 25 pg/mL and 247 ± 17 pg/mL vs. 334 ± 36 pg/mL, respectively; p < 0.05 for each comparison). Moreover, serum MCP-1 levels were significantly lower in the hyperlipidemic group after 12 months of the pravastatin treatment than in the non-hyperlipidemic group (247 ± 17 pg/mL vs. 349 ± 29 pg/mL, respectively; p < 0.05). At baseline, there was no significant difference in serum MCP-1 levels between the two patient groups of hyperlipidemia and non-hyperlipidemia (334 ± 36 pg/mL vs. 349 ± 29 pg/mL, respectively). Urinary MCP-1 levels were not significantly changed after 6 and 12 months of the pravastatin treatment relative to the baseline levels in the hyperlipidemic group (402 ± 59 ng/gCr and 354 ± 36 ng/gCr vs. 390 ± 64 ng/gCr, respectively). In addition, there was no significant difference in urinary MCP-1 levels between the two patient groups of hyperlipidemia and non-hyperlipidemia at baseline (390 ± 64 ng/gCr vs. 373 ± 78 ng/gCr, respectively). As shown in , no significant correlation was observed between serum LDL-C and MCP-1 levels in all of the data at baseline and 6 and 12 months after the pravastatin treatment in the hyperlipidemic group and at baseline in the non-hyperlipidemic group (r = 0.1112, p = 0.5003).
Figure 1. Effects of pravastatin treatment on serum and urinary MCP-1 levels in hyperlipidemic type 2 diabetic patients with normoalbuminuria. Serum and urinary MCP-1 levels were determined at baseline and 6 and 12 months following the introduction of pravastatin treatment in the hyperlipidemic diabetic patients. Serum and urinary MCP-1 levels were also measured at baseline in non-hyperlipidemic type 2 diabetic patients with normoalbuminuria. HL and non-HL indicate hyperlipidemic and non-hyperlipidemic type 2 diabetic patients with normoalbuminuria, respectively. *p < 0.05 vs. values at 0 mo in HL group; #p < 0.05 vs. values in non-HL group.
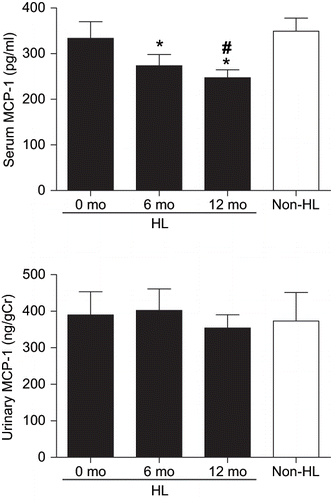
Figure 2. Correlation between serum LDL-C and MCP-1 levels in all data at baseline and 6 and 12 months following the introduction of pravastatin treatment in hyperlipidemic type 2 diabetic patients with normoalbuminuria, as well as at baseline in the non-hyperlipidemic type 2 diabetic patients with normoalbuminuria.
DISCUSSION
Experimental studies have shown that LDL-C stimulates the expression of fibronectin and MCP-1 in cultured mesangial cells,Citation[20] and also that oxidized LDL-C is cytotoxic in the kidney.Citation[21],Citation[22] Therefore, LDL-C reduction is expected to provide beneficial effects on preventing the development or progression of diabetic nephropathy. However, there is little evidence to determine whether LDL-C-lowering therapy really contributes to renoprotection in type 2 diabetic patients, especially in those with normoalbuminuria.
In the present study, treatment with 10 mg/day of pravastatin for 12 months expectedly reduced the levels of LDL-C in hyperlipidemic type 2 diabetic patients with normoalbuminuria (i.e., before the development of diabetic nephropathy). However, the pravastatin treatment did not affect the levels of urinary albumin and transferrin as markers of renal glomerular damage or urinary NAG as a marker of renal tubular injury in these patients. Therefore, we could not find a close relationship between the cholesterol-lowering effect of pravastatin and clinical markers of renal disease at the stage before the development of diabetic nephropathy. Similarly, a recent study has reported that a reduction in LDL-C level by treatment with rosuvastatin or atorvastatin for 16 weeks did not lead to a decrease in urinary albumin excretion in hyperlipidemic type 2 diabetic patients with microalbuminuria (i.e., with incipient diabetic nephropathyCitation[23]). Thus, statins may reduce LDL-C level without affecting clinical markers of diabetic nephropathy, such as urinary albumin level, before and at the stage of early diabetic nephropathy. Interestingly, the Cholesterol and Recurrent Events (CARE) study reported that pravastatin treatment reduced the decline rate of glomerular filtration rate (GFR)—in other words, renal functional loss in hyperlipidemic patients with moderate chronic renal insufficiency.Citation[24] Collectively, a reduction in LDL-C level by statin treatment may greatly contribute to renoprotection in the advanced stage rather than the early stage of diabetic nephropathy.
Type 2 diabetic patients have been shown to have chronic vascular inflammation.Citation[25] The measurement of circulating MCP-1 level seems to be a good method to assess the systemic vascular inflammation clinically in the diabetic patients. Elevated circulating MCP-1 level could reflect systemic vascular inflammation and increased vascular MCP-1 expression. Actually, recent clinical studies have indicated that elevated circulating MCP-1 level increases a risk for CVD linked closely to systemic vascular inflammation and atherosclerosis.Citation[26],Citation[27] On the other hand, increased urinary MCP-1 level could represent the enhanced degree of renal tubulointerstitial inflammation and tubular damage.Citation[17],Citation[18] By measuring levels of serum and urinary MCP-1 in addition to serum hsCRP, a widely used biomarker of systemic inflammation, we further explored whether long-term pravastatin treatment provides the anti-inflammatory effects in the whole body or locally in the kidney in hyperlipidemic type 2 diabetic patients with normoalbuminuria.
In the present study, serum MCP-1 and hsCRP levels were significantly reduced following the introduction of pravastatin treatment in hyperlipidemic type 2 diabetic patients with normoalbuminuria, indicating that the systemic inflammation in these patients was mitigated by the pravastatin treatment. Twelve months of the pravastatin treatment lowered LDL-C levels in the hyperlipidemic group to levels near to those in the non-hyperlipidemic group. Nevertheless, serum MCP-1 levels were significantly lower in the hyperlipidemic group after the pravastatin treatment than in the non-hyperlipidemic group. In addition, no significant correlation was observed between serum LDL-C and MCP-1 levels in all the data at baseline and 6 and 12 months following the introduction of pravastatin treatment in the hyperlipidemic group and at baseline in the non-hyperlipidemic group. These findings collectively suggest that pravastatin administration itself, but not LDL-C levels, may be associated with a reduction in serum MCP-1 levels in type 2 diabetic patients with normoalbuminuria. In contrast, the pravastatin treatment did not reduce urinary MCP-1 levels in the hyperlipidemic type 2 diabetic patients with normoalbuminuria. Taken together, long-term pravastatin treatment appears to contribute to the mitigation of systemic inflammation rather than local renal inflammation induced by hyperglycemia in these patients.
We conclude that long-term pravastatin treatment does not affect renal function and clinical markers of renal disease in hyperlipidemic type 2 diabetic patients with normoalbuminuria, although it effectively reduces LDL-C levels in these patients. In addition, we suggest that pravastatin may ameliorate systemic vascular inflammation rather than local renal inflammation in type 2 diabetic patients with normoalbuminuria, independent of its cholesterol-lowering effects.
ACKNOWLEDGMENTS
The authors are grateful to Sankyo Co. Ltd. for technical support. We also thank Ms. Hiromi Fujishima for her skillful technical assistance.
REFERENCES
- Turner RC, Millns H, Neil HAW, Stratton IM, Manley SE, Matthews DR, et al. Risk factor for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom prospective diabetes study (UKPDS: 23). BMJ 1998; 316: 823–828
- Betteridge DJ. Long-term risk reduction: Who needs treatment?. Diabetes Res. Clin. Pract. 2005; 68S2: S15–S22
- Danesh FR, Kanwar YS. Modulatory effects of HMG-CoA reductase inhibitors in diabetic microangiopathy. FASEB J. 2004; 18: 805–815
- Ray KK, Cannon CP. The potential relevance of the multiple lipid-independent (pleiotropic) effects of statins in the management of acute coronary syndromes. J. Am. Coll. Cardiol. 2005; 46: 1425–1433
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: A randomized placebo-controlled trial. Lancet. 2003; 360: 2005–2016
- Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): Multicentre randomized placebo-controlled trial. Lancet 2004; 364: 685–696
- Sukhova GK, Williams JK, Libby P. Statins reduce inflammation in atheroma of nonhuman primates independent of effects on serum cholesterol. Arterioscler. Thromb. Vasc. Biol. 2002; 22: 1452–1458
- Albert MA, Danielson E, Rifai N, Ridker PM. PRINCE Investigators. Effect of statin therapy on C-reactive protein levels: The pravastatin inflammation/CRP evaluation (PRINCE): A randomized trial and cohort study. JAMA 2001; 286: 64–70
- Jialal I, Stein D, Balis D, Grundy SM, Adams-Huet B, Devaraj S. Effect of hydroxymethyl glutaryl coenzyme A reductase inhibitor therapy on high sensitive C-reactive protein levels. Circulation. 2001; 103: 1933–1935
- Reape TJ, Groot PHE. Chemokines and atherosclerosis. Atherosclerosis. 1999; 147: 213–225
- Shin WS, Szuba A, Rockson SG. The role of chemokines in human cardiovascular pathology: Enhanced biological insights. Atherosclerosis. 2002; 160: 91–102
- Krishnaswamy G, Kelley J, Yerra L, Smith JK, Chi DS. Human endothelium as a source of multifunctional cytokines: Molecular regulation and possible role in human disease. J. Interferon. Cytokine. Res. 1999; 19: 91–104
- Rovin BH, Yoshimura T, Tan L. Cytokine-induced production of monocyte chemoattractant protein-1 by cultured human mesangial cells. J. Immunol. 1992; 148: 2148–2153
- Prodjosudjadi W, Gerritsma JS, Klar-Mohamad N, Gerritsen AF, Bruijn JA, Daha MR, et al. Production and cytokine-mediated regulation of monocyte chemoattractant protein-1 by human proximal tubular epithelial cells. Kidney Int. 1995; 48: 1477–1486
- Lloyd CM, Minto AW, Dorf ME, Proudfoot A, Wells TN, Salant DJ, et al. RANTES and MCP-1 play an important role in the inflammatory phase of crescentic nephritis but only MCP-1 is involved in crescent formation and interstitial fibrosis. J. Exp. Med. 1997; 185: 1371–1380
- Wada T, Furuichi K, Sakai N, Iwata Y, Yoshitomo K, Shimizu M, et al. Up-regulation of monocyte chemoattractant protein-1 in tubulointerstitial lesions of human diabetic nephropathy. Kidney Int. 2000; 58: 1492–1499
- Morii T, Fujita H, Narita T, Shimotomai T, Fujishima H, Yoshioka N, et al. Association of monocyte chemoattractant protein-1 with renal tubular damage in diabetic nephropathy. J. Diabetes Complications 2003; 17: 11–15
- Morii T, Fujita H, Narita T, Koshimura J, Shimotomai T, Fujishima H, et al. Increased urinary excretion of monocyte chemoattractant protein-1 in proteinuric renal disease. Ren. Fail. 2003; 25: 439–444
- Koshimura J, Narita T, Sasaki H, Hosoba M, Yoshioka N, Shimotomai T, et al. Urinary excretion of transferrin and orosomucoid are increased after acute protein loading in healthy subjects. Nephron Clin. Pract. 2005; 100: c33–c37
- Rovin BH, Tan LC. LDL stimulates mesangial fibronectin production and chemoattractant expression. Kidney Int. 1993; 43: 218–225
- Coritsidis G, Rifici V, Gupta S, Rie J, Shan ZH, Neugarten J, et al. Preferential binding of oxidized LDL to rat glomeruli in vivo and cultured mesangial cells in vitro. Kidney Int. 1991; 39: 858–866
- Gupta S, Rifici V, Crowley S, Brownlee M, Shan Z, Schlondorff D. Interactions of LDL and modified LDL with mesangial cells and matrix. Kidney Int. 1992; 41: 1161–1169
- Sorof J, Berne C, Siewert-Delle A, Jorgensen L, Sager P. Effect of rosuvastatin or atorvastatin on urinary albumin excretion and renal function in type 2 diabetic patients. Diabetes Res. Clin. Pract. 2006; 72: 81–87
- Tonelli M, Moye L, Sacks FM, Cole T, Curhan GC. Effect of pravastatin on loss of renal function in people with moderate chronic renal insufficiency and cardiovascular disease. J. Am. Soc. Nephrol. 2003; 14: 1605–1613
- Feng L, Matsumoto C, Schwartz A, Schmidt AM, Stern DM, Pile-Spellman J. Chronic vascular inflammation in patients with type 2 diabetes. Diabetes Care 2005; 28: 379–384
- de Lemos JA, Morrow DA, Sabatine MS, Murphy SA, Gibson CM, Antman EM, et al. Association between plasma levels of monocyte chemoattractant protein-1 and long-term clinical outcomes in patients with acute coronary syndromes. Circulation. 2003; 107: 690–695
- Deo R, Khera A, McGuire DK, Murphy SA, Meo Neto J, de P, Morrow DA, et al. Association among plasma levels of monocyte chemoattractant protein-1, traditional cardiovascular risk factors, and subclinical atherosclerosis. J. Am. Coll. Cardiol. 2004; 44: 1812–1818