Abstract
Background. Malnutrition is common in patients with chronic renal failure (CRF), and its prevalence before the initiation of dialysis is poorly characterized in these patients in developing countries. There is a paucity of data on the quantification of malnutrition and inflammation in undialyzed patients of CRF from India. This study analyzed the prevalence and causes of malnutrition in patients with CRF before the initiation of dialysis treatment. Material and Methods. In the present study, assessments of nutritional and inflammatory status were carried out in patients with CRF. Serum albumin, body mass index (BMI), triceps skin fold thickness (TST), mid-arm muscle circumference (MAMC), and subjective global assessment (SGA) scoring were used for assessment of nutritional parameters. Serum C-reactive protein and serum ferritin level were used to assess the inflammatory state of the patient. Results. Two hundred and three (146 male, 57 female) patients with CRF were included in the study from August 2004 to April 2006. Overall, the prevalence of malnutrition was 65% (131/203). The age of malnourished patients (93 male, 38 female) ranged from 11–82, with mean age of 52 ± 12.68 years. The mean serum total protein and albumin were also significantly lower in patients with malnutrition in comparison to non malnourished cases (5.50 ± 0.40 gm/dL vs. 5.74 ± 0.38 gm/dL; p < 0.05, and 3.18 ± 0.58 gm/dL vs. 3.68 ± 0.55 gm/dL; p < 0.05). The C-reactive protein and serum ferritin were significantly elevated in the malnourished group as compared to non-malnourished patients (63% vs. 33%; p < 0.05, and 301.2 ± 127.1 mg/dL vs. 212.7 ± 124.9 mg/dL; p < 0.05). Conclusion. Thus, malnutrition was common in patients with CRF before the commencement of dialysis. These data indicate that an emphasis should be placed on the assessment and prevention or correction of malnutrition in patients with CRF because of its documented adverse effect on the outcome on maintenance dialysis.
INTRODUCTION
Chronic renal failure (CRF) is an increasingly common condition worldwide. The prevalence of CRF in India varies from 0.16–0.78%.Citation[1] In developed countries, malnutrition is frequently found in patients with CRF both before and after the initiation of dialysis.Citation[2], CitationCitationCitation[5] The reported incidence of malnutrition in CKD patients is 37–84%, a percentage that increases once renal replacement therapy starts.Citation[6] The prevalence of malnutrition in CRF was found to be 43.2% in a Nigerian study.Citation[7] Common causes of malnutrition in CRF are decreased food intake, metabolic acidosis, increased serum leptin levels, hyperparathyroidism, insulin resistance, growth factor deficiency, dialysis-related nutrient loss, and membrane bioincompatibility.Citation[8] The prevalence of inflammation is very high (30–50%) in ESRD patients, and chronic inflammatory state may cause hypoalbuminemia in these patients.Citation[9],Citation[10] The metabolism of serum albumin has been adversely hampered in patients with CRF, and these include the redistribution of albumin into the interstitium, decreased synthesis, and external loss of albumin.Citation[11] Protein catabolism is further increased in patients undergoing hemodialysis with bioincompatible membranes.Citation[8]
Currently, several approaches (e.g., clinical evaluation, anthropometric measurements, biophysical and biochemical methods) have been used to assess nutrition. Serum albumin is probably still the most commonly used nutritional marker in chronic renal failure patients and is also a marker of inflammation (negative correlation) in these patients.Citation[12] Predialysis serum albumin less than 29 g/L is associated with an increased risk of progressive left ventricular hypertrophyCitation[13] and mortality in patients with CRF.Citation[14], CitationCitationCitation[17] The prevalence of malnutrition in Indian patients with CRF before the initiation of dialysis has not been characterized. This study highlights the prevalence of malnutrition in undialyzed Indian patients with CRF and its possible causes.
MATERIALS AND METHODS
Study Cohort
The study was conducted at the department of Nephrology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India, from August 2004 to April 2006. We have analyzed the nutritional status of 203 (146 male, 57 female) patients with CRF before the initiation of dialysis. The criteria for clinical diagnosis of CRF were persistently raised serum creatinine (>1.5mg %) for ≥3 months, uremic symptoms lasting for >3 months, radiological evidence of bilateral shrunken kidneys (<8.5 cm), presence of renal osteodystrophy, and broad cell cast in urine sediment. The informed consent was obtained in each case. Patients with gastrointestinal disease, nephrotic syndrome, SIRS, sepsis, shock, multiorgan failure, advanced senility, or dementia interfering with the application of the nutritional questionnaire, use of steroid or non-steroid anti-inflammatory or immunosuppressive agents, and refusal to cooperate with the study were excluded. None of the patients had a history of acute or chronic infection, such as tuberculosis, within the last three months. The study was approved by the ethical committee of Institute of Medical Sciences, Banaras Hindu University.
Assessment of Nutritional Status
The following parameters were used for the assessment of nutrition in patients with CRF.
Anthropometry
The average of three measurements of body weight, height, triceps skin fold thickness (TST), and mid-arm muscle circumference (MAMC) were measured in all patients. Body mass index (BMI) was calculated in each patients. The anthropometric parameters suggesting malnutrition were BMI (<20 kg/m2), triceps skin fold thickness (male, <6mm; female, <8mm), mid-arm muscle circumference (male, <20 cm, female, <18.5 cm).
Biochemical Indices
After an overnight fasting, blood samples were drawn and centrifuged for 4,000 cycle/minute, and serum was separated. Serum total protein, albumin, creatinine, calcium, phosphorous, total cholesterol, HDL cholesterol, and triglycerides were measured. Hemoglobin was measured by Sahli's method; serum albumin was estimated by the dye binding capacity buffer method (Vorley-1980); serum creatinine by alkaline picrate method of Jaffe's reaction; quantitative analysis using chemiluminescence with Bayers' reagent (Centaur Advia-Bayer Diagnostics), was used to access serum ferritin; and C-reactive protein (CRP) was measured using qualitative analysis (latex agglutination). Creatinine clearance was calculated using Cockcroft-Gault formulae.
Inflammatory Markers
Serum C-reactive protein and ferritin were used to assess the inflammatory state of the patient. Serum albumin ≤3.5mg/dL was defined as hypoalbuminemia, and patients with serum albumin <3.5gm/dL were classified as malnourished. Serum C-reactive protein of >0.6mg/dL was taken as a presence of inflammation. Serum ferritin>200mg/dL was considered evidence of malnutrition and inflammation as well.
Subjective Global Assessment (SGA)
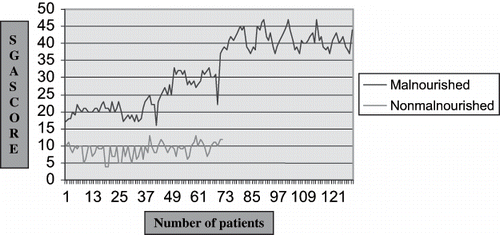
Under SGA score system, the assessment is based on the patient history and physical examination. The history consists of five criteria and focuses on weight loss in the preceding six months, gastrointestinal symptoms, dietary food intake, functional capacities, and co-morbities. The physical examination includes two items that focus on loss of subcutaneous fat and muscle wasting. A seven point scoring was applied to these seven variables. The patients classified in terms of three SGA scores: A = well nourished (score 1–14), B = moderate malnourished (score 15–35), C = severely malnourished (score 36–49). The patients with score ≥15 were taken as malnourished (see ).
Table 1 Subjective global assessment (SGA)Citation[18]
Statistical Analysis
Data were represented as mean ± standard deviation. A p < 0.05 was taken as statistically significant. A comparison between the two groups was performed by student t test (unpaired) for the various variables.
RESULTS
A total of 203 (146 male and 57 female) patients with chronic renal failure had participated in this study. Diabetic nephropathy (28%) was the most common cause of CRF, followed by chronic glomerulonephritis (24%), chronic tubulointerstitial nephritis (22%), obstructive uropathy (7.5%), and polycystic kidney disease (4%). The evidence of malnutrition was noted in 131 (male 93; female 38) patients. The mean age of patients with malnutrition was 52.99±12.68 years, with a male predominance (71%). The mean anthropometric measurements in malnourished patients were body mass index, 21.99±3.37kg/m2; triceps skin fold thickness, 8.70 ± 4.08 mm; and mid-arm muscle circumference, 22.43 ± 2.74 cm. On the basis of SGA score, malnutrition was noted in 131 patients (i.e., mild-moderate, 70 [35%]; severe, 61 [30%]); the remaining 72 (35%) patients were well nourished. Thus, evidence of malnutrition was noted in 65% of patients with CRF (see ).
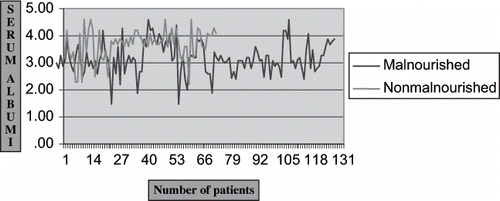
Nutritional, inflammatory, hemoglobin, and lipid profiles of patients with malnutrition (SGA-B, C) were compared with non-malnourished (SGA-A) CRF patients. Patients of CRF with malnutrition had lower BMI, TST, and MAMC in comparison to those with no obvious signs and symptoms of malnutrition (21.99 ± 3.37 Kg/m2 vs. 24.08 ± 3.85 Kg/m2, p < 0.05; 8.7 ± 4.08 mm vs. 12.91 ± 3.98 mm, p < 0.05; and 22.43 ± 2.74 cm vs. 24.65 ± 2.84 cm, p < 0.05). Serum total protein and albumin were higher in the non-malnourished CRF patients in comparison to malnourished patients (5.74 ± 0.38 gm/dL vs. 5.50 ± 0.40 gm/dL, p < 0.05; 3.68 ± 0.55 gm/dL vs. 3.18 ± 0.58 gm/dL, p < 0.05; see ). The inflammatory markers serum ferritin and C-reactive protein were elevated significantly in patients with malnutrition in comparison to those without malnutrition (301.2 ± 127.1 mg/dL vs. 212.7 ± 124.9 mg/dL, p < 0.05; 63% vs. 33%, p < 0.05). The lipid profile of both the groups (malnourished vs. non-malnourished) were compared, and serum triglyceride was the only atherosclerotic marker to be significantly increased in patients with malnutrition (161 ± 19.56 mg/dL vs. 147.2 ± 13.92 mg/dL, p < 0.05). Serum total iron binding capacity was the only marker of anaemia to be elevated significantly in well-nourished patients in comparison to the malnourished group (308.19 ± 68.48 mg/dL vs. 241.7 ± 72.34 mg/dL, p < 0.05; see ).
Table 2 Comparison of nutritional parameters in patients with malnutrition and nonmalnourished chronic renal failure
The etiology of malnutrition was multifactorial in individual patients. They included poor nutritional intake because of anorexia and vomiting related to CRF (90%), metabolic acidosis (56%), and inflammatory stress of chronic kidney disease (63%). Evidence of hyperparathyroidism (elevated intact parathyroid hormone >300 pg/mL) was noted in 21%. We also observed degree of renal insufficiency associated with malnutrition. shows the relationship between estimated glomerular filtration rate (eGFR) and nutritional status. It is evident from the table that the percentage of patients with malnutrition increases in a step wise fashion as GFR declines. Seventy-six percent of individuals with malnutrition had an eGFR of less than 15 mL/min.
Table 3 Relationship between eGFR and nutritional status
DISCUSSION
This study describes assessment of nutritional status in undialyzed patients with CRF. Malnutrition is frequent in patients with CRF, adversely affecting the quality of life associated with increased mortality and morbidity.Citation[3],Citation[19],Citation[20] Causes of malnutrition in CRF and in patients on hemodialysis are numerous and multifactorial.Citation[21],Citation[22] There is no single ideal or well-established laboratory method for the diagnosis of malnutrition in patients with CRF. Therefore, nutritional status in CRF should be assessed with combinations of valid complementary measures rather than any single measure alone. Nevertheless, the malnutrition in chronic renal failure is frequently ignored. Serum albumin is useful in identifying malnutrition, but is often abnormal late in the course of deteriorating renal function. Moreover, serum level of albumin can be confounded by concomitant liver disease, iron deficiency anemia, and chronic inflammation.Citation[8] Several methods of nutritional status evaluation are available, ranging from anthropometry measurements to more elaborate techniques such as DEXA and laboratory parameters such as albumin, prealbumin, cholesterol, and transferrin. Detzky et al. described a special methodology, named SGA (subjective global assessment), that may circumvent these problems.Citation[23] It is an easy and simple method of assessing nutrition, but unfortunately, the final assessment depends on the subjective impression of the evaluator.
Using SGA criteria,Citation[18] patients were classified as malnourished (SGA-B, C) and non-malnourished (SGA-A). The prevalence of malnutrition in the study group was 65%. The prevalence is slightly higher in our patients than in the Western world (10–54%).Citation[2],Citation[3] The poor socioeconomic conditions in the developing world and related consequences could explain the higher prevalence of malnutrition in our study population. The mean age of patients in malnourished (group I) and non-malnourished (group II) were almost equal. There was a slight male predominance in both the groups of patients. The anthropometric measurements (i.e., BMI, TST, and MAMC) of the malnourished (group I) were significantly lower than in the non-malnourished (group II) patients (see ). Thus, our observation was similar to other research.Citation[3] The reason for decreased MAMC, BMI, and TST thickness in patients with CRF are reduced calorie intake and elevated serum leptin levels, which are known to cause muscle wasting.Citation[24] Serum total protein and albumin levels in patients with CRF were significantly lower in the malnourished group of patients in comparison to the non-malnourished group (5.50 ± 0.40 gm/dL vs. 5.74 ± 0.38 gm/dL, p < 0.05; 3.18 ± 0.58 gm/dL vs.3.68 ± 0.55 gm/dL, p < 0.05). This corroborates the finding in Nigerian CRF patientsCitation[7] and suggests that serum albumin is a clinical marker of malnutrition in CRF patients. A higher level of serum creatinine was noted in patients with malnutrition, suggesting that patients with advanced renal failure have a higher prevalence of malnutrition in comparison to patients with moderate-severe renal failure. Renal insufficiency was strongly associated with malnutrition independent of relevant demographic, social, and medical condition in adults.Citation[25] This supports the belief that renal insufficiency itself is a catabolic and inflammatory condition. Garg et al. observed that 31% of individuals with malnutrition had GFR < 60 mL/min/1.73m2. In a multivariate analysis, a GFR of <30 mL/min/1.73m2 was independently associated with malnutrition after adjustment for relevant demographic, social, and medical conditions.Citation[25] Thus, renal insufficiency is an important independent risk factor for malnutrition. In the present study, we observed that eGFR of patients with malnutrition was much lower in comparison to non-malnourished cases.
The inflammatory marker, C-reactive protein, was increased in 83 (63%) patients with malnutrition in comparison to only 24 (33%; p < 0.05) cases of non-malnourished patients. Another inflammatory marker, serum ferritin, was significantly elevated in patients with malnutrition in comparison to those without malnutrition (301.2 ± 127.1 mg/dL vs. 212.7 ± 124.9 mg/dL, p < 0.05). Although there have been several studies to support the evidence of inflammatory stress in patients on dialysis,Citation[26], CitationCitation[28] there haven't been any isolated studies to suggest inflammatory stress in undialyzed CRF patients. The prevalence of inflammation varies from 30–75% in CKD patients, depending on multiple factors such as residual renal function, geographies, genetic differences, dialysis therapy, and comorbidities, as well as the cutoff point used for the diagnosis of inflammation by CRP.Citation[29],Citation[30] The reasons for the increased prevalence of persistent low grade inflammation in ESRD patients are complex and include a variety of factors related to uremia—such as decreased clearance of cytokines, oxidative stress, accumulation of advanced glycation end products, and infectious complications—and dialysis-related factors—such as membrane bioincompatibility, vascular access infections, and endotoxin exposure—that stimulates the inflammatory response by activating the production of IL-1, IL-6, TNF-α, and interferon-γ by the macrophages.Citation[30] In addition, the impaired immune response coupled with a persistent immune stimulation might have an important role in the low-grade inflammation and altered cytokine balance that is present in ESRD.Citation[31] Serum TIBC was elevated in patients without apparent signs and symptoms of malnutrition. The most likely reason for the above finding is iron deficiency, resulting in decreased iron store and low serum transferrin level. We also found a significant increase in serum triglyceride in patients with malnutrition in comparison to the non-malnourished group. The presence of elevated serum triglyceride levels further heightens the already increased cardiovascular risk in malnourished CRF patients. Similar observations were reported in a recent publication from Nigeria.Citation[7] The causes of malnutrition in patients with CRF are complex and multifactorial. It appears that several factors in combinations contribute to the development of malnutrition and wasting syndrome in patients with CRF.Citation[6],Citation[8] We have observed that anorexia related to uremia was the most common (90%) cause of malnutrition. The other causes included inflammatory stress (63%), metabolic acidosis (56%), and hyperparathyroidism in 21% of patients. Inflammatory stress of CRF may contribute to malnutrition and wasting.Citation[32]
In summary, malnutrition and inflammation are highly prevalent and often coexist in undialyzed Indian patients with chronic renal failure. The early detection of malnutrition and appropriate nutritional supplements are required to treat malnutrition and inflammation in CRF patients.
ACKNOWLEDGMENT
This work was presented in XLIV European Renal Association-European Dialysis and Transplantation Association (ERA-EDTA) Congress, 21–24 June, 2007, Barcelona, Spain.
REFERENCES
- Agarwal SK. Chronic kidney disease and its prevention in India. Kidney Int Suppl. 2005; 98: S41–S45
- Kopple JD. Pathophysiology of protein-energy wasting in chronic renal failure. J Nutr. 1999; 129: S247–S251
- Lawson JA, Lazarus R, Kelly JJ. Prevalence and prognostic significance of malnutrition in chronic renal insufficiency. J Ren Nutr. 2001; 11: 16–22
- Hakim RM, Levin N. Malnutrition in hemodialysis patients. Am J Kidney Dis. 1993; 21: 125–137
- Pupim LB, Ikizler TA. Uremic malnutrition: New insights into an old problem. Semin Dial. 2003; 16: 224–232
- Stevinkel P, Lindholam B, Heimburger O. Novel approaches in an integrated therapy of inflammatory-associated wasting in end stage renal disease. Semin Dial. 2004; 17(6)505–515
- Agaba EI, Agaba PA. Prevalence of malnutrition in Nigerians with chronic renal failure. International Urology and Nephrology. 2004; 36: 89–93
- Toigo G, Aparicio M, Attman PO, et al. Expert Working Group report on nutrition in adult patients with renal insufficiency. Part 1 of 2. Clinical Nutrition. 2000; 19(3)197–207
- Stenvinkel P. Inflammatory and atherosclerotic interactions in depleted uremic patients. Blood Purify. 2001; 19: 53–61
- Kaysen GA, Rathore V, Shearer GC, Depner TA. Mechanisms of hypoalbuminemia in hemodialysis patients. Kidney Int. 1995; 48: 510–516
- Kaysen GA. Hypoalbuminemia in dialysis patients. Semin Dial. 1996; 9: 249–256
- Stenvinkel P, Barany P, Chung SH, Lindholm B, Heimbürger O. A comparative analysis of nutritional parameters as predictors of outcome in male and female ESRD patients. Nephrology Dialysis Transplant. 2002; 17: 1266–1274
- Moon KH, Song IS, Yang WS, et al. Hypoalbuminemia as a risk factor for progressive left ventricular hypertrophy in hemodialysis patients. Am J Nephrol. 2000; 20: 396–401
- Lowrie EG, Lew NL. Death risk in hemodialysis patients: The predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis. 1990; 15: 458–482
- Avram MM, Mittman N, Bonomini L, Chattopadhgay J, Fein P. Markers for survival in dialysis: A seven-year prospective study. Am J Kidney Dis. 1995; 26: 209–219
- Leavey SF, Strawderman RL, Jones CA, Port FK, Held PJ. Simple nutritional indicators as independent predictors of mortality in hemodialysis patients. Am J Kidney Dis. 1998; 31: 997–1006
- Fung F, Sherrard DJ, Gillen DL, et al. Increased risk for cardiovascular mortality among malnourished end-stage renal disease patients. Am J Kidney Dis. 2002; 40: 307–314
- Sacks GS, Dearman K, et al. Use of subjective global assessment to identify nutrition-associated complications and death in geriatric long-term care facility residents. J Am Coll Nutr. 2000; 19(5)570–577
- Hakim RM, Levin N. Malnutrition in hemodialysis patients. Am J Kidney Dis. 1993; 21: 125–137
- Don BR, Kaysen GA. Assessment of inflammation and nutrition in patients with end-stage renal disease. J Nephrol. 2000; 13: 249–259
- Kalantar-Zadeh K, Ikizler TA, Block G, Avram MM, Kopple JD. Malnutrition-inflammation complex syndrome in dialysis patients: causes and consequences. Am J Kidney Dis. 2003 Nov; 42(5)864–881
- http:/www.blackwell-synergy.com/Links/doi.htm, Wolfson M. Pathogenesis and treatment of malnutrition in maintenance dialysis. 2000.
- Detzky AS, McLaughlin JR, Baker JP, et al. What is subjective global assessment of nutritional status?. Journal of Paraenteral Nutrition. 1987; 11: 8–13
- Mak RH, Cheung W, Cone RD, Marks DL. Leptin and inflammation-associated cachexia in CKD. Kidney Int. 2006; 69: 794–798
- Garg AX, Blake PG, Clark WF, et al. Association between renal insufficiency and malnutrition in older adults: Results from the NHANES III. Kidney Int. 2001; 60(5)1867–1874
- Kaysen GA, Dubin JA, Muller HG, Mitch WE, Rosales LM, Levin NW. Relationships among inflammation nutrition and physiologic mechanisms establishing albumin levels in hemodialysis patients. Kidney Int. 2002; 61: 2240–2249
- Kalantar-Zadeh K, Kopple JD. Relative contributions of nutrition and inflammation to clinical outcome in dialysis patients. Am J Kidney Dis. 2001; 38: 1343–1350
- Kaysen GA. Malnutrition and the acute-phase reaction in dialysis patients—how to measure and how to distinguish. Nephrol Dial Transplant. 2000; 15: 1521–1524
- Kaysen GA. The microinflammatory state in uremia: Causes and potential consequences. J Am Soc Nephrol. 2001; 12: 1549–1557
- Stevinkel P, Alvestrand D. Inflammation in end-stage renal disease: Sources, consequences, and therapy. Semin Dial. 2002; 15(5)329–337
- Stenvinkel P, Ketteler M, Johnson RJ, et al. IL-10, IL-6, and TNF-α: Central factors in the altered cytokine network of uremia—the good, the bad, and the ugly. Kidney Int. 2005; 67(4)1216–1233
- Avesani CM, Carrero JJ, Axelsson J, et al. Inflammation and wasting in chronic kidney disease: Partners in crime. Kidney Int. 2006; 70: S8–S13