Abstract
Background. Atherosclerotic vascular change affecting the lower extremities is the most common peripheral vascular disease. Ankle-brachial index (ABI) and toe-brachial index (TBI) are common, non-invasive diagnostic tests for atherosclerosis in the lower extremities. Peritoneum is a vascular-based structure. The use of glucose-based hyperosmolar solutions for PD patients results in a significant increase in blood glucose load and can be considered atherogenic. The association between ABI or TBI values and peritoneal function in patients undergoing peritoneal dialysis remains unclear. We presumed that the risk factors for atherosclerosis in large and small vessels may differ. Methods. A total of 146 peritoneal dialysis patients, 41 males and 105 females (119 without diabetes and 27 with diabetes), received peritoneal dialysis for more than four months. Patients who had dialysis-related peritonitis within six months prior to this study were excluded. The ABI or TBI was determined using an automated, non-invasive, waveform analysis device. Results. The ABI value correlated positively with mean arterial pressure and TBI value. The TBI value correlated positively with ABI value and inversely with fasting serum glucose and serum total cholesterol concentrations. Peritoneal function was not correlated with ABI or TBI. Conclusion. This cross-sectional study demonstrated that risk factors in peritoneal dialysis patients for atherosclerosis in large vessels and small vessels differed. Interestingly, peritoneal function test is not associated with ABI or TBI value. However, further investigation of the association between ABI or TBI value and cardiovascular events is required for this patient group.
INTRODUCTION
Peritoneal dialysis (PD) is widely utilized for treating patients with end-stage renal disease (ESRD). The peritoneal membrane is composed of a layer of mesothelial cells, underlined by an electro-negative basement and loose connective tissue. Transport across the peritoneal membrane is linked to vascular surface area and the intrinsic permeability of the peritoneum, which is determined by permeability of the capillary in the peritoneal membrane.Citation[1] Peritoneal dialysis impairs both the structure and function of the peritoneal membrane. Histological changes of the peritoneal membrane and peritoneal vessels have been described,Citation[2–5] particularly subendothelial vascular membrane damage in vessels.Citation[6] Peripheral vascular disease is more common in patients with end-stage renal disease (ESRD). Atherosclerosis performs as an intimal lesion disturbing conduit functions and occurs via narrowing arteries causing ischemia or infarction of an organ or tissue.Citation[7] Atherosclerotic change in the peripheral artery is termed peripheral arterial occlusive disease (PAOD). Atherosclerotic vascular disease affecting the lower extremities is the most common form of peripheral vascular disease.Citation[8] Atherosclerosis is also a chronic low-grade inflammatory disease with particular cellular and molecular responses.Citation[9] Factors associated with atherosclerosis include advanced age, hypertension, smoking, uremia, and diabetes mellitus. Additionally, a relationship between hypoalbuminemia and peripheral vascular disease has been identified in ESRD patients undergoing hemodialysis.Citation[10] Ankle-brachial (ABI) and toe-brachial (TBI) indexes are simple, effective tools for clinical use when assessing peripheral vascular disease. A reduction in the ABI or TBI index is correlated with generalized atherosclerosis. These tests exploit the fact that PAOD generates selective reductions in systolic blood pressure in the lower extremities.
The use of glucose-based hyperosmolar solutions for PD patients results in a significant increase in blood glucose load. This metabolic effect results in a persistent tendency for patients undergoing PD to develop obesity, hyperglycemia, and hyperlipidemia.Citation[11] Glucose loading can be considered atherogenic. We believe that atherosclerosis develops in central large vessels and in small peripheral vessels such as peritoneal vessels, and we presumed that the risk factors for atherosclerosis in large and small vessels may differ. This study also investigates the relationship between the value of the ABI and TBI indices and peritoneal function to determine whether peritoneal function is a factor predictive of arterial stiffness in PD patients.
MATERIALS AND METHODS
Study Population
This cross-sectional study enrolled 146 patients, 41 males and 105 females, who had undergone continuous ambulatory peritoneal dialysis (CAPD) for at least four months. Patients who had dialysis-related peritonitis within six months prior to this study were excluded. All patients were recruited between June 6, 2005, and September 30, 2005. The selection of inclusion criteria was based on the desire to identify any correlations between possible changes to peritoneal function and vascular degeneration. Baxter CAPD solution was used for PD (Baxter Healthcare SA, Singapore). Blood samples for serum albumin concentrations, calcium, inorganic phosphate, intact parathyroid hormone (i-PTH), serum glucose, triglyceride, and total cholesterol concentrations were drawn routinely under a fasting status during the same study month. Six subjects were taking aspirin, and one subject was taking clopidogrel. Patients were considered to have diabetes mellitus when they were diagnosed with diabetes mellitus or when they used insulin/oral anti-diabetic drugs. Smoking frequency, alcohol intake, and vascular disease, including coronary artery, cerebrovascular, and peripheral artery disease, were assessed via a questionnaire.
Body height was measured with participants standing in bare feet, and weight was measured with the subjects wearing light clothes. Systolic and diastolic blood pressures were measured with a Vascular Profiler 1000 (VP-1000) (Colin Corporation, Japan). Body mass index was calculated by entering body weight and height into the same machine and was calculated as weight (kg) divided by height squared (m2). Peritoneal equilibration test (PET) and mass transfer area coefficient for creatinine (MTAC Cr) were presented as peritoneal function. PET was performed as described by Twardowski et al Citation[12] According to dialysate/plasma creatinine ratio (D/P Cr) at 4 hours of standard PET, peritoneal transport types were classified into four types: low, low-average, high-average, or high. Moreover, MTAC Cr was calculated using the Garred equation:Citation[13]where V0 = initial infusion dialysate volume, V4 = final drained out dialysate volume at 4 hours (240 minutes), P = plasma concentration of creatinine, D0 = initial dialysate concentration of creatinine, and D4 was the final dialysate concentration of creatinine at 240 minutes.
Assessment of the ABI and TBI
The ABI and TBI were measured using a Vascular Profiler 1000 (VP-1000) (Colin Corporation, Japan). The ABI was determined as the ratio of ankle systolic blood pressure to brachial systolic blood pressure. The TBI was the ratio of big toe systolic blood pressure to brachial systolic blood pressure. The highest left and right brachial systolic pressures were used for calculating the ABI and TBI. Personal data (birth date, height, weight, and gender) were input into the profiler. This device simultaneously records brachial-ankle pulse wave velocity (baPWV), systolic blood pressure, diastolic blood pressure, pulse pressure, electrocardiogram, and phonocardiogram. After resting for at least 10 minutes, subjects were placed in a supine position, and the auto-calculated value for both the ABI and TBI were used for statistical analysis. Electrocardiographic electrodes were attached to both wrists, and a microphone for phonocardiography was placed at the third intercostal space at the left margin of the sternum. Pneumatic pressure cuffs were placed snugly around arms ankles. Toe blood pressure was measured using a 25 mm-wide digital cuff around the base of both big toes.
Statistical Analysis
Data are expressed as means ± standard deviations. Pearson correlation coefficient was applied to examine the univariate association between ABI or TBI and study variables. Step-wise multiple linear regression analysis was performed to identify which variables were independently correlated with ABI and TBI. Dependent variables were ABI and TBI, and independent variables were mean arterial blood pressure, age, body mass index, calcium-phosphate products (Ca×P), intact parathyroid hormone (i-PTH), serum glucose concentration, serum albumin concentration, serum total cholesterol concentration, serum triglyceride concentration, MTAC, male gender, smoking, ABI or TBI, and dialysis duration. Between-group comparisons were performed using the Student's t-test. Least-significant difference (LSD) one-way analysis of variance (ANOVA) was performed to compare the ABI or TBI values of the various peritoneal transport types. Statistical significance was p < 0.05. All statistical analyses utilized the Statistical Package for the Social Sciences (SPSS) V12.0 for Windows (SPSS Inc., USA).
RESULTS
Patient Characteristics
In total, 146 subjects were enrolled in this study. shows the subject characteristics. Mean age was 48±11 years. Mean daily exchange volume was 8731±1770 ml, and mean ultrafiltration volume was 1238±517 ml. Seven subjects had ABI <0.9, and 7 subjects had TBI < 0.6. Thirty-four patients had anuria, and 27 patients (18%) had diabetes. Both ABI and TBI were not different between males and females (ABI, 1.08±0.2 VS. 1.08±0.1, p = 0.979; TBI, 0.78±0.13 VS. 0.80±0.14, p = 0.569). A greater proportion of males than females smoked and drank alcohol. Fourteen subjects had diabetes mellitus. Seven patients had atherosclerotic disease, including: two (1%) with cerebrovascular disease, three (2%) with coronary arterial disease, and five (3%) with peripheral vascular disease. Factors significantly different between males and females were total cholesterol level (182.8±40.6 vs. 214.3±49.0, p < 0.001) and triglyceride level (164.0±141.0 vs. 263.3±208.9, p = 0.005).
Table 1 Characteristics of the peritoneal dialysis patients
Ankle-Brachial Index and Toe-Brachial Index
In this study, ABI was correlated with TBI (p < 0.001, r = 0.335). presents the correlation between ABI and TBI. lists the coefficients of correlation in Pearson correlation analysis between ABI and TBI and other clinical variables. Analytical results demonstrated that hemodynamic variables and serum calcium level correlated with ABI, and serum glucose, serum total cholesterol, and serum triglyceride levels correlated with TBI. By step-wise multiple regression analysis, ABI and TBI values were used as dependent variables, and mean arterial blood pressure, age, body mass index, calcium-phosphate products (Ca×P), intact parathyroid hormone (i-PTH), MTAC, serum glucose concentration, serum albumin concentration, serum total cholesterol concentration, serum triglyceride concentration, male gender, smoking, ABI or TBI, and dialysis duration were used as independent variables. shows the results of step-wise multiple regression analysis between ABI and TBI and other clinical variables.
Figure 1. Relationship between ankle-brachial index (ABI) and toe-brachial index (TBI) in peritoneal dialysis patients.
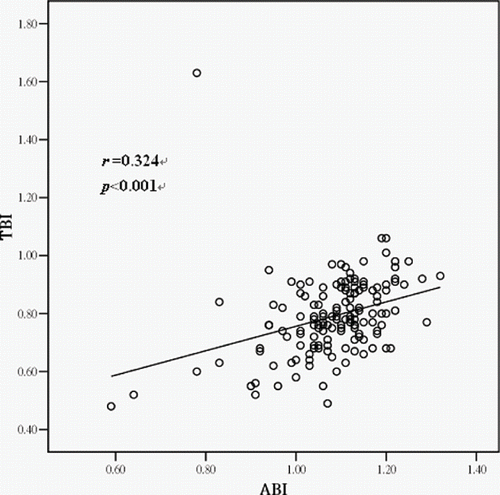
Table 2 Correlation coefficients between ABI/TBI and other variables
Table 3 Results of step-wise multiple regression analysis to assess the correlation of ABI or TBI with other variables
The following formula was utilized to determine correlations between ABI and TBI and independent variables for all patients:where
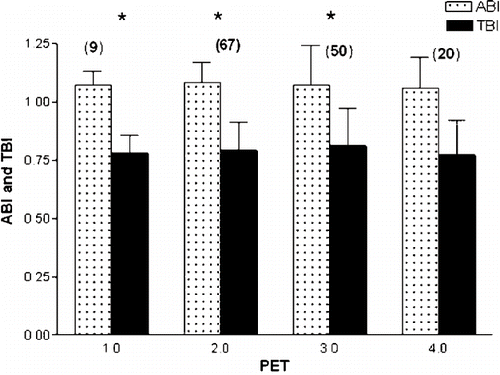
To further examine the possible influence of PET on atherosclerosis, one-way ANOVA was performed using peritoneal transport type as an independent factor. In this study, the low transport group contained 9 patients (ABI, 1.07±0.06; TBI, 0.78±0.08), the low-average transport group contained 67 patients (ABI, 1.08±0.09; TBI, 0.79±0.12), the high-average transport group contained 50 patients (ABI, 1.07±0.17; TBI, 0.81±0.16), and the high transport group contained 20 patients (ABI, 1.06±0.13; TBI, 0.77±0.15). shows that ABI or TBI values did not differ among the four study groups.
Figure 2. No significant difference of ankle-brachial index (ABI) and toe-brachial index (TBI) in patients with different peritoneal equilibration test (PET) category: 1 = low, 2 = low average, 3 = high average, 4 = high. *p > 0.05 vs. group 4. The number in parentheses indicates the number of patients in each group.
DISCUSSION
This study investigated the feasibility using ABI and TBI for classic cardiovascular risk assessment and determining peritoneal function in patients undergoing peritoneal dialysis. This is the first study to demonstrate that ABI is independently correlated with mean arterial blood pressure and TBI value, and that TBI is independently correlated with ABI, fasting serum glucose, and serum total cholesterol concentrations in PD patients.
Ankle-Brachial Index
Chuang et al. reported that low ABI was significantly correlated with advanced age, high systolic blood pressure, high diastolic blood pressure, high pulse pressure, and smoking.Citation[14] They also demonstrated that the prevalence of atherosclerosis was very low for male and female patients aged <70 years, and that males had lower ABI values than females aged >70 years. Newmen et al.Citation[15] demonstrated that no gender difference existed in for ABI for subjects ages >70 years. They also demonstrated that in old population, low ABI was correlated with advanced age, smoking, diabetes mellitus, high systolic blood pressure, low diastolic blood pressure, low body mass index, and high total cholesterol and high triglyceride concentrations. This study identified that male gender is not important to ABI. One possibility is that in this study, mean age of the subjects (48±11 years) was younger than those age in a previous study.Citation[14]
Although hypertension is a risk factor for atherosclerosis among patients without ESRD, the relationship between hypertension and ABI or TBI values in patients undergoing PD has not been reported. In this study, a positive correlation existed between ABI value and systolic blood pressure, diastolic blood pressure, and pulse pressure. In a longitudinal study of the general population,Citation[16] among traditional risk factors, blood pressure was not an independent factor or significant predictor for ABI values. Vinuesa et al.Citation[17] described that in chronic kidney disease patients, low ABI value was correlated with low systolic blood pressure, low diastolic blood pressure, and high pulse pressure. Ono et al.Citation[18] identified a significant relationship between low ABI value and low diastolic blood pressure and high pulse pressure. Interestingly, Cheung et al.Citation[19] determined that conventional cardiovascular risk factors, such as male gender, hyperlipidemia, and hypertension, were not associated with PAOD. Therefore, it is likely that the correlation between hypertension and PAOD is obscure.
This study found no correlation between serum lipid and ABI values, which is consistent with findings for studies of a hemodialysis population,Citation[18] diabetic population,Citation[20],Citation[21] and general population.Citation[14] However, Newman et al. demonstrated that hyperlipidemia was associated with low ABI value in an elderly population (77±6 years).Citation[15] Thus, it seems likely that old age (> 70 years) may alter the correlation between serum lipid and ABI values.
Toe-Brachial Index
Aboyans et al.Citation[16] recently identified risk factors contributing differentially to the progression of large-vessel peripheral vascular disease (LV-PAD) and small-vessel peripheral vascular disease (SV-PAD). Their study of the general population demonstrated that cigarette smoking, elevated lipid levels, and inflammation contribute to LV-PAD, and that diabetes was the only significant predictor of SV-PAD progression. No study has identified these relationships for patients undergoing PD. Similar to the study by Aboyans el al., this study demonstrated that the different risk factors affect both ABI and TBI values. Chronic low-grade inflammation has an important role in the pathogenesis of atherosclerosis.Citation[9]. In patients with type 2 diabetes, the TBI was more strongly correlated than ABI with serum high-sensitivity C-reactive protein (hsCRP), and plasma fibrinogen, both of which are indicators of vascular inflammation.Citation[21] However, in ESRD patients, the sensitivity of ABI measurements of PAOD in patients with ESRD is likely less sensitive than that for the general population due to the high prevalence of arterial calcification. Notably, TBI is more appropriate than ABI tests for screening patients with ESRD for PAOD.Citation[22]
In this cross-sectional study, diabetic patients had low TBI values (0.7 vs. 0.8, p = 0.01), and hypercholesterolemia subjects had low TBI value (0.74 vs. 0.8, p < 0.01). In studies of patients with type 2 diabetes, TBI was low in hypercholesterolemia patients,Citation[20] whereas Aso et al.Citation[21] described that no relationship existed between hypercholesterolemia and TBI. A recent study provided evidence that in general population, diabetes was the only significant predictor of reduced TBI.Citation[16] The same result was obtained by Haltmayer et al.Citation[23] They demonstrated that smoking and high plasminogen levels are related to atherosclerosis of proximal segments, and diabetes to that of distal segments. However, in this study, all subjects using glucose-based hyperosmolar solutions had a tendency to develop obesity, hyperglycemia and hyperlipidemia. It is clear that diabetes mellitus is the major predictor of atherosclerosis in digital segments. This study showed that long-term glucose loading, such as that in PD patients, has the same result for diabetic patients.
This study has several limitations, such as that it is a cross-sectional observational study. This study had significantly more females than males (41 males and 105 females). However, among clinical parameters, triglyceride and total cholesterol levels were significantly different between male and females. When a cut-off value of 240 mg/dL was employed to divide hyper- and normal total cholesterol levels, no significant difference was found.
In conclusion, this study confirmed the effect of blood pressure and TBI value on the ABI value and the impact of fasting serum glucose, ABI value, and serum total cholesterol levels on the TBI value. Interestingly, this study determined that neither ABI nor TBI was correlated with peritoneal function in patients undergoing PD. The association between ABI and TBI values and cardiovascular events in PD patients has not been published and warrants further investigation.
ACKNOWLEDGMENTS
The authors would like to thank the Colin Medical Technology Corporation in Taiwan for financially supporting this research. Members of the peritoneal dialysis center at Chang Gung Memorial Hospital are appreciated for their technical assistance.
REFERENCES
- Davies SJ, Williams JD. Peritoneal dialysis: Principles, techniques, and adequacy. Comprehensive Clinical Nephrology, RJ Johnson, J Fehally. Mosby, LondonUK 2003; 1003–1004
- Topley N, Coles GA, Williams JD. Biocompatibility studies on peritoneal cells. Perit Dial Int. 1994; 14(Suppl. 3)S21–S28
- Dobbie JW, Anderson JD, Hind C. Long-term effects of peritoneal dialysis on peritoneal morphology. Perit Dial Int. 1994; 14(Suppl. 3)S16–S20
- Hendriks PM, Ho-dac-Pannekeet MM, van Gulik TM, et al. Peritoneal sclerosis in chronic peritoneal dialysis patients: analysis of clinical presentation, risk factors, and peritoneal transport kinetics. Perit Dial Int. 1997; 17(2)136–143
- Mateijsen MA, van der Wal AC, Hendriks PM, et al. Vascular and interstitial changes in the peritoneum of CAPD patients with peritoneal sclerosis. Perit Dial Int. 1999; 19(6)517–525
- Bertoli SV, Buzzi L, Ciurlino D, Maccario M, Martino S. Morpho-functional study of peritoneum in peritoneal dialysis patients. J Nephrol. 2003; 16(3)373–378
- O'Rourke M. Mechanical principles in arterial disease. Hypertension. 1995; 26: 2–9
- Weitz JI, Byrne J, Clagett GP, et al. Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: A critical review. Circulation. 1996; 1;94(11)3026–3049
- Russell R. Mechanism of disease: Atherosclerosis—an inflammatory disease. N Eng J Med. 1999; 340(2)115–126
- O'Hare AM, Hsu CY, Bacchetti P, Johansen KL. Peripheral vascular disease risk factors among patients undergoing hemodialysis. J Am Soc Nephrol. 2002; 13(2)497–503
- Davies SJ, Williams JD. Peritoneal dialysis: principles, techniques, and adequacy. Comprehensive Clinical Nephrology, RJ Johnson, J Fehally. Mosby, LondonUK 2003; 1023
- Twardowski ZJ, Nolph KD, Khanna R, et al. Peritoneal equilibtation test. Perit Dial Bull. 1987; 7: 138–147
- Garred LJ, Canaud B, Farrell PC. A simple kinetic model for assessing peritoneal mass transfer in chronic ambulatory peritoneal dialysis. ASAIO J. 1982; 6: 131–137
- Chuang SY, Chen CH, Cheng CM, Chou P. Combined use of brachial-ankle pulse wave velocity and ankle-brachial index for fast assessment of arteriosclerosis and atherosclerosis in a community. Int J Cardiol. 2005; 98(1)99–105
- Newman AB, Sutton-Tyrrell K, Vogt MT, Kuller LH. Morbidity and mortality in hypertensive adults with a low ankle/arm blood pressure index. JAMA. 1993; 28(270[4])487–489
- Aboyans V, Criqui MH, Denenberg JO, et al. Risk factors for progression of peripheral arterial disease in large and small vessels. Circulation. 2006; 113(22)2623–2629
- de Vinuesa SG, Ortega M, Martinez P, et al. Subclinical peripheral arterial disease in patients with chronic kidney disease: Prevalence and related risk factors. Kidney Int. 2005; 93: S44–S47
- Ono K, Tsuchida A, Kawai H, et al. Ankle-brachial blood pressure index predicts all-cause and cardiovascular mortality in hemodialysis patients. J Am Soc Nephrol. 2003; 14(6)1591–1598
- Cheung AK, Sarnak MJ, Yan G, et al. Atherosclerotic cardiovascular disease risks in chronic hemodialysis patients. Kidney Int. 2000; 58(1)353–362
- Aso Y, Okumura K, Wakabayashi S, et al. Elevated pregnancy-associated plasma protein-A in sera from type 2 diabetic patients with hypercholesterolemia: Associations with carotid atherosclerosis and toe-brachial index. J Clin Endocrinol Metab. 2004; 89(11)5713–5717
- Aso Y, Okumura K, Inoue T, et al. Results of blood inflammatory markers are associated more strongly with toe-brachial index than with ankle-brachial index in patients with type 2 diabetes. Diabetes Care. 2004; 27(6)1381–1386
- O'Hare A, Johansen K. Lower-extremity peripheral arterial disease among patients with end-stage renal disease. J Am Soc Nephrol. 2001; 12(12)2838–2847
- Haltmayer M, Mueller T, Horvath W, et al. Association between erythrocyte mean corpuscular volume and peripheral arterial disease in male subjects: A case control study. Angiology. 2001; 52(9)605–613