Abstract
Background. Brachial-ankle pulse wave velocity (baPWV), a non-invasive waveform analysis, is a useful tool for the vascular evaluation of arterial stiffness. Increased baPWV values have been found increased in patients with arterial stiffness. The aim of this study was to investigate retrospectively the association between arterial stiffness and the common medications used in peritoneal dialysis patients. Methods. In all, 116 peritoneal dialysis patients (35 males and 81 females) received peritoneal dialysis for more than four months. Patients who had dialysis-related peritonitis or other infection within six months prior to this study, inflammation disease, fasting serum sugar ≥126 mg/dL, and/or use of oral hypoglycemic agents or insulin injections were excluded. The medications that were enrolled in our study were calcium-containing phosphate binders, vitamin-B complex, folic acid, and antihypertensive medications. baPWV was determined using an automated, non-invasive, waveform analysis device. Results. In a step-wise multiple linear regression analysis, baPWV correlated independently with systolic blood pressure (t = 8.4, p < 0.001) and age (t = 5.5, p < 0.001), and inversely correlated with body mass index (t = −3.19, p = 0.002) and the use of angiotensin II receptor antagonists (t = −2.35, p = 0.021). Conclusion. In this retrospective study of peritoneal dialysis patients, we found that angiotensin II receptor antagonists (ARBs) in peritoneal dialysis patients may be an independent factor for arterial stiffness. Hence, we suggest that compared with other antihypertensive drugs, ARBs may be a good choice for lowering arterial stiffness in PD patients. However, further studies on the optimal treatment of arterial stiffness in peritoneal dialysis patients are warranted.
INTRODUCTION
Preclinical arterial changes are often associated with arteriosclerosis and atherosclerosis. Arteriosclerosis occurs in the degeneration of the arterial media and decreases the cushioning function of arteries, a condition called arterial stiffness.Citation[1] Factors associated with arterial stiffness may include uremia, hypertension, advanced age, and diabetes mellitus. Furthermore, some studiesCitation[2],Citation[3] have identified a relationship between hypoalbuminemia and arterial stiffness in ESRD patients undergoing hemodialysis. As we know, the use of glucose-based hyperosmolar solutions for peritoneal dialysis patients results in a significant increase in the glucose load. This metabolic effect is a persistent tendency for patients undergoing peritoneal dialysis to develop obesity, hyperglycemia, and hyperlipidemia. However, arterial stiffness is commonly asymptomatic, making assessment difficult. A previous study demonstrated that ESRD patients have stiffer arteries than the the non-uremic population.Citation[4] Hence, the incidence of cardiovascular disease is higher in patients with ESRD undergoing renal replacement therapy than in the general population. In this line, pulse wave velocity (PWV), measured by pulse transit time and distance between the carotid and femoral artery, provides an index of arteriosclerosis.Citation[5] However, aortic PWV measurement may be less appropriate, as it is relatively time-consuming due to the inaccessibility of the central arteries. A new automatic noninvasive apparatus, brachial-ankle pulse wave velocity (baPWV), has recently been developed, and measurement with this apparatus has good compatibility with carotid femoral pulse wave velocity.Citation[6]
Calcium-containing phosphate binders, folic acid, vitamins, and antihypertensive medications are commonly used in ESRD patients. In addition, there is evidence that the rennin-angiotensin system contributes to oxidative stress and vascular remodeling,Citation[7] which has an important role for arterial stiffness. In previous study,Citation[8] folic acid supplementation reduced arterial stiffness. The aim of this study was to investigate the relationship between arterial stiffness and the traditional risk factors and common medications in peritoneal dialysis patients.
MATERIALS AND METHODS
Study Population
This study initially enrolled 147 patients (42 males and 105 females) who had undergone continuous ambulatory peritoneal dialysis for at least four months. Patients who had dialysis-related peritonitis or infection disease within six months prior to this study, inflammation disease, a fasting serum sugar ≥ 126 mg/dL, and/or use of anti-diabetic drugs or insulin injections were excluded. In all, 116 subjects were enrolled in our study (35 males and 81 females). All patients were recruited between June 6, 2005, and September 30, 2005. Our reason for selecting these inclusion criteria was to avoid the vascular effect by diabetes mellitus, hyperglycemia, systemic inflammation, and infection. Peritoneal dialysis supplies came from Baxter CAPD solution (Baxter Healthcare SA, Singapore Branch). Blood samples for serum albumin concentrations, serum glucose, and total cholesterol concentration were drawn in a fasting status in the same month of this study. Smoking frequency and alcohol intake were assessed using a questionnaire.
The medications that were enrolled in our study were calcium containers (calcium carbonate, 500 mg/tab; calcium acetate, 667 mg/tab), vitamin-b complex, folic acid (5 mg/tab), angiotensin II receptor antagonists (ARB; losartan, 50 mg/tab; irbesartan, 150 mg/tab; valsartan, 80 mg/tab), angiotensin-converting enzyme inhibitors (ACEI; enalapril, 5 mg/tab; captopril, 25 mg/tab; fosinopril, 10 mg/tab), β-blockers (carvedilol, 25, 6.25 mg/tab; nadolol, 80 mg/tab; labetalol, 200 mg/tab; bisoprolol, 5 mg/tab; propranolol, 40 mg/tab), and calcium-channel blockers (nifedipine, 30 mg/tab; felodipine, 5 mg/tab; amlodipine, 5 mg/tab; diltiazem, 30 mg/tab). All of them were recoded from medical charts. In Mahmud's study,Citation[9] two months of therapy with losartan decreased aortic stiffness and arterial wave reflection. According to Mahmud's study, our subjects who had used the above antihypertensive drugs for more than two months prior the baPWV study were enrolled in the positive medication group. In some research,Citation[10–12] 4-week, 12-week, and 2-year treatment with different dosages of folic acid were assayed. Hence, we chose the mean daily dose or mean daily number of tablets within six months prior to the baPWV study to represent the condition of calcium containers, vitamin-b complex, and folic acid.
Body height was measured with participants in bare feet and weight as measured wearing light clothes. Systolic and diastolic blood pressures were measured with the Vascular Profiler 1000 (VP-1000) (Colin Corporation, Japan). Body mass index was also calculated by entering body weight and height into the same machine. Body mass index was calculated as weight (kg) divided by the square of height (m²).
Assessment of baPWV
Brachial-ankle pulse wave velocity was measured using a Vascular Profiler 1000 (VP-1000; Colin Corporation, Japan). Personal data (birthday, height, weight, and gender) were input into the profiler. After at least 10 minutes of rest, study subjects were placed in a supine position, and the auto-calculated value of baPWV were used for statistical analysis. This device simultaneously records baPWV, systolic blood pressure, diastolic blood pressure, pulse pressure, electrocardiogram, and phonocardiogram. Pneumatic pressure cuffs were placed snugly around arms and ankles. Electrocardiographic electrodes were attached to both wrists, and a microphone for phonocardiography was placed at the third intercostal space at the left margin of the sternum. baPWV was calculated according to the following equation:where D1 is the distance between heart and ankle, D2 is the distance between heart and brachium, and t is the transit time between brachial arterial waves and tibial arterial waves. These distances were calculated automatically according the subject height. The baPWV of 113 patients among the 116 patients (97%) were measured by one operator. The difference in interoperator measurement of baPWV was 2.1%. The difference in intraoperator measurements of baPWV was 2.2%.
Statistical Analysis
In this study, data are expressed as means ± standard deviations. Differences between two groups were evaluated with Student's t-test and chi-square test. Step-wise multiple linear regression analysis was performed to determine which variables were independently correlated with baPWV. For the purpose of this analysis, the dependent variable was baPWV and independent variables were systolic blood pressure, age, body mass index, serum albumin concentration, serum total cholesterol concentration, male gender, dialysis duration, alcohol consumption, smoking, and the use of angiotensin II receptor antagonists (ARB), angiotensin-converting enzyme inhibitors (ACEI), β-blockers, calcium-channel blockers, folic acid, calcium containers, and vitamin b complex. Least-significant difference (LSD) one-way analysis of variance (ANOVA) was used to compare the results of the blood pressure (systolic, diastolic, and pulse pressure) of the different antihypertensive medication groups. Statistical significance was p < 0.05. All statistical analyses utilized Statistical Package for the Social Sciences (SPSS) V12.0 for Windows (SPSS Inc., USA).
RESULTS
Patient Characteristics
Finally, 116 subjects were enrolled in this study. Mean age was 48 ± 10 years; mean dialysis duration was 52 ± 3 months. Twenty-five patients had anuria. Nine subjects had been smoking, and six subjects had consumed alcohol. presents the characteristics for study subjects. baPWV was not different for male and female (1652 ± 338 cm/s vs. 1794 ± 497 cm/s, p = 0.13). A greater proportion of men had habits of smoking and alcohol drinking. Among the clinical parameter, only total cholesterol level (185 ± 41 vs. 212 ± 49, p = 0.005) was different for males and females in our study. Five patients had atherosclerotic disease (including 0.7% cerebrovascular disease, 2% coronary arterial disease, and 0.7% peripheral vascular disease). In this study, thirty-one patients had used ARBs, forty-one patients had used β-blockers, and fifty-six patients had used calcium-channel blockers.
Table 1 Characteristics of the peritoneal dialysis patients
Brachial-Ankle Pulse Wave Velocity and Other Clinical Parameters
In a step-wise multiple regression analysis, we used the baPWV value as a dependent variable and systolic blood pressure, age, gender, BMI, serum albumin concentration, serum total cholesterol concentration, duration of dialysis, alcoholic consumption, smoking, mean daily dosage of calcium supplements, mean daily tablets of vitamin-b complex, mean daily dose of folic acid, history of ARBs, history of β-blockers, and history of calcium-channel blockers as independent variables. depicts the result of stepwise multiple regression analysis between baPWV and other clinical variables. Systolic blood pressure and age were potent significant variables for baPWV, and body mass index and the taking of ARBs were significant inverse variables for baPWV.
Table 2 Results of step-wise multiple regression analysis to assess the correlation of baPWV with other variables
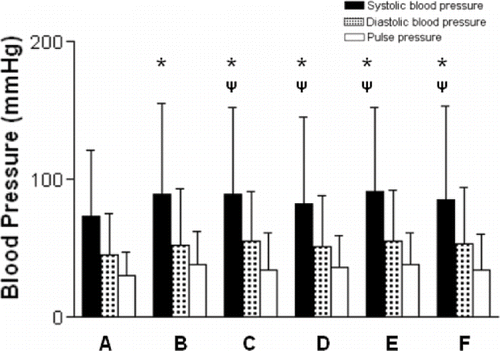
To further explore the possible role of antihypertensive effects by the medications on the arterial stiffness, we performed one-way ANOVA test using kinds of antihypertensive drugs as independent factor. By the history of antihypertensive medication, we classified the patient into six groups:
Group A: 41 subjects without medication;
Group B: 5 subjects using ARBs only;
Group C: 3 subjects using β-blockers only;
Group D: 18 subjects using calcium-channel blockers only;
Group E: 28 subjects using ARBs combining calcium-channel blocker or β-blocker or both; and
Group F: 21 subjects using calcium-blocker and β-blocker.
depicts that blood pressures (systolic, diastolic, and pulse pressure) were not different among five groups of those using different kinds of antihypertensive drugs in the present study.
Figure 1. No significant difference of blood pressures (systolic, diastolic, pulse pressure) in patients with different kinds of antihypertensive medications (i.e., group A, without hypertension and no antihypertensive medications; group B, using ARBs; group C, using β-blockers; group D, using calcium-channel blockers. group E, using ARBs combing calcium-channel blockers or β-blockers or both; group F, using Ca-blockers and β-blockers). *p < 0.05 vs. group A; ψ: p > 0.05 vs. group B.
DISCUSSION
The present study has examined the feasibility of baPWV measurement for classic cardiovascular risk assessment and common drugs undergoing peritoneal dialysis. We have demonstrated for the first time that baPWV is independently correlated with ARB-taking, systolic blood pressure, age, and body mass index in patients undergoing peritoneal dialysis. Hence, in peritoneal dialysis patients, the arterial stiffness marker baPWV was independently correlated with ARB-taking after adjusting for other established cardiovascular risk factors.
The present study demonstrated that duration of peritoneal dialysis was not significantly related to baPWV. Our results interestingly contrast with a previous study reported by Suzuki et al.Citation[13] in which duration of hemodialysis was one of the most significant variables affecting baPWV in maintenance hemodialysis patients, although the specific reasons for the difference are not clear. As we know, the correlation between peritoneal dialysis and arterial stiffness is not discussed popularly. Peritoneal dialysis versus hemodialysis is the plausible difference.
Chuang et al. found that in one community, baPWV was significantly related to age, systolic blood pressure, fasting blood levels of glucose, and creatinine values.Citation[14] They also demonstrated that men had higher baPWV than women until around 60 years old. Nagano et al.Citation[15] demonstrated that no gender difference was found in the baPWV because of the high mean age. The present study found that gender is not a variable for baPWV. One possibility is that in Chuang's study,Citation[14] the mean age of men was higher than the mean age of women (58.2 ± 11.3 vs. 55.3 ± 10.8, p < 0.0001). In the present study, the mean age of men was younger than the mean age of women, though it was not significantly different (47 ± 11 vs. 48 ± 10, p = 0.543).
In the present study, body mass index was negatively associated with baPWV. This result is consistent with the report in general population in previous study.Citation[15] However, another study presented a positive association between BMI and arterial stiffness in the general population.Citation[14] As we know, no study has yet mentioned the correlation between arterial stiffness and BMI in peritoneal dialysis patients. However, in hemodialysis patients, BMI had not been found to significant correlate with arterial stiffness.Citation[3] Shinohara et al.Citation[16] found no correlation between BMI and aortic pulse wave velocity in a healthy population, those with renal failure before starting hemodialysis, and those undergoing hemodialysis. Given this overall picture, it seems likely that the correlation between BMI and arterial stiffness is obscure.
The main question of this study was whether supplement ARBs reduces arterial stiffness in peritoneal dialysis patient. Recently, there were several studies that found that angiotensin II receptor blocker inhibit the progression of arterial stiffness,Citation[17],Citation[18] the mechanism of which remains undefined. A more active rennin-angiotensin-aldosterone system may contribute to enhanced oxidative stress and vascular remodeling.Citation[7],Citation[19] Ichihara et al.Citation[17] showed that in the presence or absence of change for blood pressure, angiotensin II receptor blocker was associated with significantly lower odds for arterial stiffness in hemodialysis patients. As we know, no study has proved any correlation between arterial stiffness and antihypertensive medications in PD patients. Interestingly, we observed that recent (two months previous to) ARB taking, compared to other antihypertensive medications, is inversely correlated to the baPWV value in the absence of change for blood pressure. However, low correlation coefficient (standard β: −0.17) was noted between the arterial stiffness and ARBs used in our study. The legitimate reason for this appearance may be that the duration of ARB taking of those enrolled in our study is shorter than the duration of other study.Citation[17],Citation[18]
The other question addressed by this study was whether supplement of folic acid reduces arterial stiffness in peritoneal dialysis patient. As we know, homocysteine is an independent risk factor for cardiovascular disease in end stage renal disease patients,Citation[20] and folic acid is commonly used in ESRD patients. Plasma homocysteine levels are effectively lowered by folic acid.Citation[21] However, that lower homocysteine can lead to a reduction in cardiovascular disease is still not enough evidence to make definite recommendations.Citation[22] In patients undergoing peritoneal dialysis, 1 mg/day folic acid supplement is recommended.Citation[23] Jassen et al.Citation[21] described that the dialysis patients on six weeks of 5 mg folic acid per day and the dialysis patients that were treated with 2.5 mg folic acid daily had similar homocysteine level. Woodside et al.Citation[24] and Van Dijk et al.Citation[25] described that arterial stiffness is not associated with total homocysteine concentration in healthy populations. This finding agrees well with that in another study by Van Guldener et al.Citation[10] in ESRD patients. Williams et al.Citation[8] found that folic acid reduced arterial stiffness, and that this response did not significantly correlate with either homocysteine or folate plasma concentrations. In the present study, average daily dose of folic acid within six months was not an independent factor for arterial stiffness in PD patients. Hence, it seems likely that in PD patients, the dosage of folic acid is not an important factor for arterial stiffness.
Although the increase of arterial stiffness is multifactorial, the exact mechanisms are not clear. In the present study, we were unable to establish a correlation between arterial stiffness and blood cholesterol alteration. Some studiesCitation[3],Citation[13],Citation[15],Citation[26] presented no correlation between total cholesterol levels and arterial stiffness. However, Wilkinson et al. reported that aortic pulse wave velocity was significantly higher in patients with hypercholesterolemia.Citation[27] In contrast, Dart et al. demonstrated that cholesterol concentration was negatively associated with thoracic aortic beta index.Citation[28] From above, it seems likely that hypercholesterolemia is not an important variable for increased arterial stiffness in our study.
We are aware of some limitations in this study, such as retrospective observation and a female predominant study group. In our study, female predominance (35 males and 81 females) is noted in the study group. However, among the clinical parameters, only total cholesterol level shows a significant difference between male and female (184 mg/dL vs. 212 mg/dL). That is, as was found in several previous studies,Citation[3],Citation[27],Citation[28] the role of total cholesterol level in arterial stiffness is not important, and such difference in gender could be ignored.
In conclusion, this study has demonstrated that increased baPWV, when reflecting upon arterial stiffness, is positively correlated with systolic blood pressure and age, and negatively correlated with body mass index and recent two months ARB taking in patients undergoing peritoneal dialysis. Hence, we suggest that compared with other antihypertensive medications, ARBs may be a good choice for lowing arterial stiffness in PD patients, though further studies on the optimal treatment of arterial stiffness in peritoneal dialysis patients are warranted.
ACKNOWLEDGMENTS
This work was supported by Colin Medical Technology Corporation in Taiwan. The authors thank the members of peritoneal dialysis center of Chang Gung Memorial Hospital for their excellent and dedicated assistance.
REFERENCES
- O'Rourke M. Mechanical principles in arterial disease. Hypertension. 1995; 26: 2–9
- Nitta K, Akiba T, Uchida K, et al. Left ventricular hypertrophy is associated with arterial stiffness and vascular calcification in hemodialysis patients. Hypertens Res. 2004; 27: 47–52
- Guerin AP, London GM, Marchais SJ, et al. Arterial stiffening and vascular calcifications in end-stage renal disease. Nephrol Dial Transplant. 2000; 15: 1014–1021
- London GM, Marchais SJ, Safar ME, et al. Aortic and large artery compliance in end-stage renal failure. Kidney Int. 1990; 37: 137–142
- Halloc P. Arterial elasticity in man in relation to age as evaluated by the pulse wave velocity method. Arch Intern Med. 1934; 54: 770–798
- Kubo T, Miyata M, Minagoe S, et al. A simple oscillometric technique for determining new indices of arterial distensibility. Hypertens Res. 2002; 25: 351–358
- Romero JC, Reckelhoff JF. Role of angiotensin and oxidative stress in essential hypertension. Hypertension. 1999; 34(4)943–949
- Williams C, Kingwell BA, Burke K, et al. Folic acid supplementation for 3 wk reduces pulse pressure and large artery stiffness independent of MTHFR genotype. Am J Clin Nutr. 2005; 82(1)26–31
- Mahmud A, Feely J. Effect of angiotensin II receptor blockade on arterial stiffness: Beyond blood pressure reduction. Am J Hypertens. 2002; 15(12)1092–1095
- van Guldener C, Lambert J, ter Wee PM, et al. Carotid artery stiffness in patients with end-stage renal disease: No effect of long-term homocysteine-lowering therapy. Clin Nephrol. 2000; 53(1)33–41
- Dierkes J, Domrose U, Ambrosch A, et al. Response of hyperhomocysteinemia to folic acid supplementation in patients with end-stage renal disease. Clin Nephrol. 1999; 51(2)108–115
- van Dijk RA, Rauwerda JA, Steyn M, Twisk JW, Stehouwer CD. Long-term homocysteine-lowering treatment with folic acid plus pyridoxine is associated with decreased blood pressure but not with improved brachial artery endothelium-dependent vasodilation or carotid artery stiffness: A two-year, randomized, placebo-controlled trial. Arterioscler Thromb Vasc Biol. 2001; 21(12)2072–2079
- Suzuki T, Yonemura K, Maruyama Y, et al. Impact of serum parathyroid hormone concentration and its regulatory factors on arterial stiffness in patients undergoing maintenance hemodialysis. Blood Purif. 2004; 22: 293–297
- Chuang SY, Chen CH, Cheng CM, et al. Combined use of brachial-ankle pulse wave velocity and ankle-brachial index for fast assessment of arteriosclerosis and atherosclerosis in a community. Int J Cardiol. 2005; 98: 99–105
- Nagano M, Nakamura M, Sato K, et al. Association between serum C-reactive protein levels and pulse wave velocity: a population-based cross-sectional study in a general population. Atherosclerosis. 2005; 180: 189–195
- Shinohara K, Shoji T, Tsujimoto Y, et al. Arterial stiffness in predialysis patients with uremia. Kidney Int. 2004; 65: 936–943
- Ichihara A, Hayashi M, Kaneshiro Y, et al. Low doses of losartan and trandolapril improve arterial stiffness in hemodialysis patients. Am J Kidney Dis. 2005; 45(5)866–874
- Agata J, Nagahara D, Kinoshita S, et al. Angiotensin II receptor blocker prevents increased arterial stiffness in patients with essential hypertension. Circ J. 2004; 68(12)1194–1198
- Schiffrin EL, Park JB, Pu Q. Effect of crossing over hypertensive patients from a beta-blocker to an angiotensin receptor antagonist on resistance artery structure and on endothelial function. J Hypertens. 2002; 20(1)71–78
- Bostom AG, Shemin D, Verhoef P, et al. Elevated fasting total plasma homocysteine levels and cardiovascular disease outcomes in maintenance dialysis patients. A prospective study. Arterioscler Thromb Vasc Biol. 1997; 17(11)2554–2558
- Janssen MJ, van Guldener C, de Jong GM, et al. Folic acid treatment of hyperhomocysteinemia in dialysis patients. Miner Electrolyte Metab. 1996; 22(1–3)110–114
- Bircher G. Nutrition in chronic renal failure. Comprehensive Clinical Nephrology, RJ Johnson, J Fehally. Mosby, LondonUK 2003; 940
- Gokal R, Harty J. Nutrition and peritoneal dialysis. Handbook of Nutrition and the Kidney, WE Mitch, S Klahr. Lippincott-Raven, Washington, DC 1998; 284
- Woodside JV, McMahon R, Gallagher AM, et al. Total homocysteine is not a determinant of arterial pulse wave velocity in young healthy adults. Atherosclerosis. 2004; 177(2)337–344
- van Dijk RA, Rauwerda JA, Steyn M, et al. Long-term homocysteine-lowering treatment with folic acid plus pyridoxine is associated with decreased blood pressure but not with improved brachial artery endothelium-dependent vasodilation or carotid artery stiffness: A two-year, randomized, placebo-controlled trial. Arterioscler Thromb Vasc Biol. 2001; 21(12)2072–2079
- Blacher J, Demuth K, Guerin AP, et al. Influence of biochemical alterations on arterial stiffness in patients with end-stage renal disease. Arterioscler Thromb Vasc Biol. 1998; 18: 535–541
- Wilkinson IB, Prasad K, Hall IR, et al. Increased central pulse pressure and augmentation index in subjects with hypercholesterolemia. J Am Coll Cardiol. 2002; 39(6)1005–1011
- Dart AM, Lacombe F, Yeoh JK, et al. Aortic distensibility in patients with isolated hypercholesterolemia, coronary artery disease, or cardiac transplant. Lancet. 1991; 338(8762)270–273