Abstract
The administration of antineoplastic agents are associated with various vascular events. A case of acute bilateral renal infarction in a patient with non-small cell lung carcinoma during chemotherapy with cisplatin and gemcitabine is reported. The patient was misdiagnosed as having renal colic. To our knowledge, bilateral renal infarction following cisplatin and gemcitabine has not been reported previously. Renal infarction should be considered in the differential diagnosis of flank pain in a patient treated with gemcitabine and cisplatin.
INTRODUCTION
The administration of antineoplastic agents are associated with various vascular events that range from simple phlebitis to lethal vascular events.Citation[1] Cisplatin, a platinum-containing agent, and gemcitabine, a pyrimidine nucleoside antimetabolite, are usually administered in combination to treat a variety of malignancies and appear to be most active in the treatment of non-small cell lung cancer.Citation[2],Citation[3] Hitherto hepatic, cutaneous, and ocular veno-occlusive diseases, Raynaud's phenomenon, myocardial infarction, cerebral ischemia, thrombotic microangiopathy, hemolytic-uremic syndrome, arterial distal thrombosis, pulmonary embolism, iliac artery embolism, and deep venous thrombosis in upper and lower limbs have been reported with cisplatin and/or gemcitabine therapy.Citation[1],Citation[4],Citation[5] A case of acute bilateral renal infarction in a patient with non-small cell lung carcinoma during chemotherapy with cisplatin and gemcitabine is reported here. This is the first reported case of acute bilateral renal infarction caused by cisplatin and gemcitabine.
CASE
A 46-year-old female patient was referred from the medical oncology department to the nephrology department because of a bilateral renal infarction image in computed tomography (CT). The patient has a three month history of non-small cell lung cancer. A chemotherapy regimen consisting of intravenous gemcitabine (1000mg/m2 days 1, 8, and 15) and intravenous cisplatin (100 mg/m2 day 1) every four weeks was followed, and local external irradiation therapy was applied to the thoracal region. In her history, after the second course of chemotherapy, she was admitted to the emergency service because of sudden onset bilateral flank pain.
On admission to emergency service, her physical examination revealed tenderness to percussion of the kidneys, but otherwise normal findings. Her intense flank pain subsided only with high dose and prolonged usage of narcotic analgesia. Record of the laboratory analyses provided the following results: hemoglobin, 12.8 g/dL; leukocyte, 4.2 × 109/L; platelet, 560 × 109/L; blood urea nitrogen, 8.1 mg/dL; creatinine, 0.89 mg/dL; lactate dehydrogenase, 604 U/L (normal 240–480); alanine aminotranspherase (ALT), 10 U/L; and aspartate aminotranspherase (AST), 21 U/L. Urinalysis showed 86 erythrocytes and 1–2 leukocytes per high-power field, and her urine density was 1005. The electrocardiogram showed no signs of arrhythmia or myocardial infarction. Direct urinary system graph, chest x-ray, and abdominal ultrasound were unremarkable.
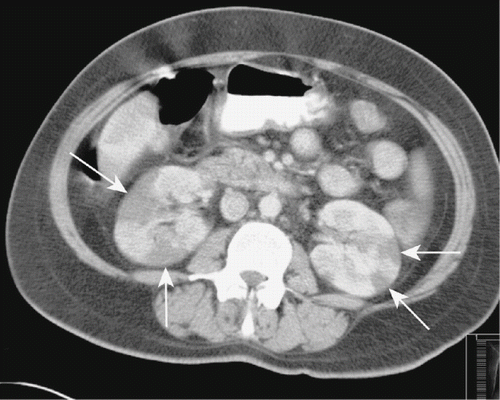
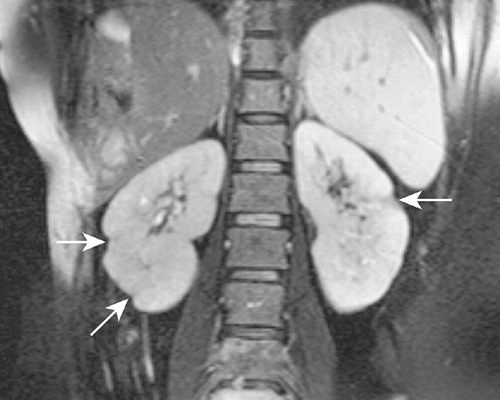
The patient was diagnosed as having ureteral colic. By day 3, her pain resolved. One day later, the patient was evaluated by CT examination before the third course of chemotherapy. CT imaging of the abdomen showed several revealed multiple wedge-shaped, sharply marginated, low-density lesions without contour bulging in both kidneys, suggestive of segmental renal infarction areas (see ). On admission to nephrology, her physical examination did not show any abnormalities. Laboratory analyses provided the following results: hemoglobin, 12.6 g/dL; platelet, 400 × 109/L; leukocyte, 6.0 × 109/L; erythrocyte sedimentation rate, 56 mm/h; blood urea nitrogen, 7.4 mg/dL; creatinine, 0.8 mg/dL; ALT, 10 U/L; AST, 25 U/L; and lactate dehydrogenase, 500 U/L. Urinalysis showed one erythrocyte and four leukocytes per high-power field, and her urine density was 1010. In 24 hours, urine collection there was 0.425 g protein. The glomerular filtration rate was estimated at 96 mL/min/1.73m2. Tests for anti-cardiolipin antibodies, antineutrophil cytoplasmic antibodies, antinuclear antibody, and anti-dsDNA were all negative. Screening test for lupus anticoagulant was 35.08 s (normal 31–44). Coagulation test provided the following results: prothrombin time, 11.23 s (range 11.23–14.44, INR ratio 0.89); activated partial thromboplastin time, 25.06 s (normal 25.8–33.2); fibrinogen, 5.78 g/dL (normal 1.75–4); anti-thrombin III activity, 113.88% (normal 75–125%); protein C activity, 115.44% (normal 70–140%); and protein S activity, 100.32% (normal 58–127.5%). Other laboratory parameters were within normal ranges. Echocardiography was normal (without evidence of intramural thrombus or valvular pathology). The electrocardiogram showed a sinus rhythm. The patient was diagnosed as having bilateral renal infarction and therapy with aspirin (100 mg/day), and ACE inhibitor (enalapril 5 mg/day) was begun. Gemcitabine and cisplatin was discontinued. The patient was evaluated by magnetic resonance (MR) imaging in her follow-up, which showed that the segmental infarction areas healed with scarring (see ). During one-month follow-up, her renal function was normal, and physical examination did not reveal any abnormality.
DISCUSSION
This appears to be the first case that reported bilateral renal infarction in a lung cancer patient treated with cisplatin and gemcitabine. Renal infarction should be considered in the differential diagnosis of flank pain in a lung cancer patient treated with gemcitabine and cisplatin. The diagnosis of renal infarction can be delayed and may misdiagnose as renal colic.
The most common causes of renal infarction are renal artery embolism secondary to mitral stenosis, vegetations in bacterial endocarditis, atrial fibrillation, thrombus in the left ventricle, and recent myocardial infarction.Citation[6–10] In the present case, we performed some laboratory analysis to elicit the etiology of the renal infarcts. We excluded the classical causes of renal infarction by laboratory analysis and medical history of the patient. In this case, we suspected that the cause of renal infarction was antineoplastic agents. Cisplatin and gemcitabine are usually administered in combination to treat a variety of malignancies, such as in the treatment of non-small cell lung carcinoma.Citation[2],Citation[3] Cisplatin and gemcitabine treatment may induce vascular events.Citation[1],Citation[4],Citation[5],Citation[11] It was shown that cisplatin increases platelet reactivity.Citation[11] Gemcitabine has been demonstrated to cause immune-mediated side effects.Citation[1],Citation[4] Drug-induced endovascular damage, platelet activation, an abnormality of thromboxane-prostacyclin homeostasis, hypomagnesemia-related arterial constriction, and endothelial damage are some of the putative mechanisms of these vascular events.Citation[1],Citation[4] However, the mechanisms of vascular events in cancer patients are largely unknown. The release of procoagulant factors by tumor cells may play role in vascular events.
In the literature, the incidence of renal infarction was reported to be 0.007%–1.4%.Citation[7–l9] Acute renal infarction may manifest clinically as a pain similar to renal colic, and is often misdiagnosed as an acute pyelonephritis or renal colic because of similar presenting symptoms, as shown in the present case.Citation[6],Citation[12] Also, in this case, we suspected tumor metastases of bilateral kidneys. An elevated serum lactate-dehydrogenase with little or no rise in serum aminotransferases is strongly suggestive of renal infarction, as seen in the present case.Citation[6],Citation[7] Proteinuria and leucocytosis may also be seen. The classic radiological finding of renal infarction is a wedge-shaped perfusion defect without any mass effect in the adjacent parenchyma and without contour bulging.Citation[13],Citation[14] The radiological findings of the presented case were compatible with multiple segmental renal infarctions. The diagnosis was confirmed by follow-up imaging, as the hypodense areas on CT at the time of diagnosis were seen to heal with scarring on MR images one month after the initial diagnosis.
In conclusion, the use of gemcitabine and cisplatin should be considered a cause of renal infarction. Furthermore, renal infarction should be considered in the differential diagnosis of flank pain in a lung cancer patient treated with gemcitabine and cisplatin.
REFERENCES
- Shahab N, Haider S, Doll DC. Vascular toxicity of antineoplastic agents. Semin Oncol. 2006; 33: 121–138
- Artal-Cortes A, Martinez-Trufero J, Herrero A, et al. Increasing-dose gemcitabine plus low-dose cisplatin in metastatic non-small cell lung cancer. Anticancer Drugs. 2003; 14: 111–118
- Metro G, Cappuzzo F, Finocchiaro G, Toschi L, Crino L. Development of gemcitabine in non-small cell lung cancer: The Italian contribution. Ann Oncol. 2006; 17: S37–S46
- Numico G, Garrone O, Dongiovanni V, et al. Prospective evaluation of major vascular events in patients with non-small cell lung carcinoma treated with cisplatin and gemcitabine. Cancer. 2005; 103: 994–999
- Barcelo R, Lopez-Vivanco G, Mane JM, Rubio I, Munoz A, Fernandez R. Distal ischemic changes related to combination chemotherapy with cisplatin and gemcitabine: Description of four cases. Ann Oncol. 2000; 11: 1191–1194
- Chu PL, Wei YF, Huang JW, Chen SI, Chu TS, Wu KD. Clinical characteristics of patients with segmental renal infarction. Nephrology. 2006; 11: 336–340
- Korzets Z, Plotkin E, Bernheim J, Zissin R. The clinical spectrum of acute renal infarction. Isr Med Assoc J. 2002; 4: 781–784
- Bolderman R, Oyen R, Verrijcken A, Knockaert D, Vanderschueren S. Idiopathic renal infarction. Am J Med. 2006; 119: 9–12
- Domanovits H, Paulis M, Nikfardjam M, et al. Acute renal infarction: Clinical characteristics of 17 patients. Medicine (Baltimore). 1999; 78: 386–394
- Carey HB, Boltax R, Dickey KW, Finkelstein FO. Bilateral renal infarction secondary to paradoxical embolism. Am J Kidney Dis. 1999; 34: 752–755
- Togna GI, Togna AR, Franconi M, Caprino L. Cisplatin triggers platelet activation. Thromb Res. 2000; 99: 503–509
- Davutoglu V, Yildirim C, Gunay N, Turkmen S. Renal infarction mimicking renal colic in patient with a prosthetic aortic valve. Emerg Med J. 2005; 22: 595–596
- Wong WS, Moss AA, Federle MP, Cochran ST, London SS. Renal infarction: CT diagnosis and correlation between CT findings and etiologies. Radiology. 1984; 150: 201–205
- Suzer O, Shirkhoda A, Jafri SZ, Madrazo BL, Bis KG, Mastromatteo JF. CT features of renal infarction. Eur J Radiol. 2002; 44: 59–64