Abstract
Fenofibrate, a fibric acid derivative, is used to treat diabetic dyslipidemia, hypertriglyceridemia, and combined hyperlipidemia alone or in combination with statins. Rhabdomyolysis is defined as a pathological condition of skeletal muscle cell damage leading to the release of toxic intracellular material into the circulation. Its major causes include trauma, ischemia, toxins, metabolic disorders, infections, and drugs. Rhabdomyolysis associated with fenofibrate is extremely rare. In nearly all of the presented cases, there was a predisposing factor for rhabdomyolysis such as diabetes, older age, renal insufficiency, and hypothyroidism. Here, we report a nondiabetic, nonhypothyroidic young female patient without any known prior renal disease presenting with acute renal failure developing after fenofibrate treatment.
INTRODUCTION
The term rhabdomyolysis refers to the disintegration of striated muscle, which results in the release of muscular cell constituents into the extracellular fluid and the circulation.Citation[1] Clinically, the syndrome presents with severe muscular pain, weakness, and myoglobinuria. Its major causes include trauma, ischemia, drugs, toxins, metabolic disorders, and infections.Citation[1],Citation[2] Acute renal failure is the most common complication of rhabdomyolysis, occurring in 10–40% of the patients.Citation[2]
Fenofibrate, a fibric acid derivative, is frequently used to treat diabetic dyslipidemia and hypertriglyceridemia alone or in combination with statins. The major effects of fibrates are to lower plasma triglyceride (TG) and increase high density lipoprotein-cholesterol (HDL-C), both substantially.Citation[3]
Here, we report a non-diabetic young female patient without any known prior renal disease presenting with acute renal failure developing after fenofibrate treatment.
CASE
A 42-year-old female was admitted to our hospital in February 2007 complaining of new onset diffuse myalgia and weakness. Four weeks before presentation, she was initiated on fenofibrate 250 mg and candesartan silexitil + hydrocholorothyazide16/12.5 mg because of hypertension and hyperlipidemia. Before this therapy, her serum creatinine was 0.8 mg/dL; alanine aminotransferase (ALT), 20 U/L; aspartate aminotransferase (AST), 16 U/L; TG, 435 mg/dL; total cholesterol, 201 mg/dL; HDL-C, 31 mg/dL; and creatinine phosphokinase (CPK), 55 U/L. She had a progressively increasing myalgia for the last 15 days, and showed dark colored urine in the emergency unite. She denied having any recent viral illness, trauma, exercise, epilepsy, or over-the-counter medication use, nor any other medication known to induce rhabdomyolysis.
Past medical history was insignificant other than hypertension lasting for seven years. She had no history of tobacco or alcohol use.
Physical examination was unremarkable except for diffuse generalized muscle pain and tenderness. There was no evidence of cardiac event. Her blood pressure was 170/90 mm Hg and pulse 88/minute. She didn't have any fever.
Urinalysis was positive for red blood cells, hemoglobin, and myoglobin. Urinary ultrasound revealed grade 1 parenchymal renal disease of normal sized kidneys. The initial laboratory results are presented in
Table 1 Initial laboratory values of the patients
After she was admitted to the hospital with a presumptive diagnosis of acute renal failure secondary to rhabdomyolysis induced by fenofibrate, all medications were discontinued. Her blood pressure was maintained with amlodipine 10 mg.
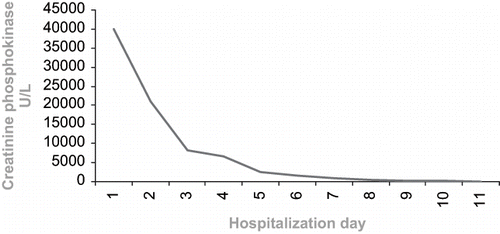
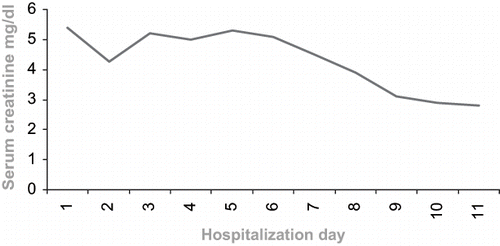
Following hydration, diuretic, and bicarbonate therapy, the patient's myalgia resolved. Although her CPK peaked to >40 000 units/I, it returned to baseline within ten days (see ). Her renal function also improved gradually within days of hospitalization without any dialysis (see ). Urine output ranged between 3–5 liters/day. She was discharged with complete recovery.
DISCUSSION
Fibrates bind to peroxisome proliferator-activated nuclear receptors-alfa with subsequent stimulation of fatty acid oxidation and a reduction in the rate of hepatic lipid generation. The major effects of fibrates are to lower plasma triglyceridemia and increase HDL-C, both substantially. Triglyceridemia may be by decreased 20–50%, and HDL-C may be increased by 10–30%.Citation[3],Citation[4]
The fibrates are generally well tolerated. Side effects are fairly uncommon and include upper gastrointestinal system disturbances, headache, anxiety, fatigue, sleep disorders, myalgia, and loss of libido.Citation[3]
Advanced age, diabetes, hypothyroidism, female sex, concomitant medications, hepatic renal insufficiency, excess alcohol intake, exercise, trauma, and surgery are all associated with higher rates of adverse effects.Citation[3],Citation[5],Citation[6]
Rhabdomyolysis is a syndrome characterized by muscle necrosis and the release of intracellular muscle contents into the systemic circulation.Citation[1] Acute renal failure, oliguric or nonoliguric, is the most common complication of rhabdomyolysis, occurring in 10–40% of patients.Citation[2] Rhabdomyolysis also accounts for 5–25% of all cases of acute renal failure.Citation[7],Citation[8]
The mechanism of antilipidemic agent-induced myopathy remains unclear. Endocrine, metabolic, and genetic factors might play a role in the pathophysiology. Generally, rapid remission of symptoms can be achieved after the discontinuation of therapy.Citation[6] The symptoms of our patient also resolved in a week after discontinuation of therapy.
The occurrence of reversible myopathy with the elevation of CPK appears to be a class effect; although it is rare, it can lead to life-threatening rhabdomyolysis. The immediate consequences of rhabdomyolysis include hyperkalemia, which may cause fatal cardiac dysrhythmia and hypocalcemia due to calcium-binding by damaged muscle proteins and phosphate.Citation[6] These adverse effects increase when the patients are treated concomitantly with statins and fenofibrate or statins and gemfibrozil and cyclosporine.Citation[9],Citation[10] Our patient had no other medication prescribed other than fenofibrate with ARB. There is no evidence in the literature regarding an angiotensin receptor blocker increasing the incidence of rhabdomyolysis.
In 252,460 patients treated with lipid-lowering agents, as only 24 cases were hospitalized with rhabdomyolysis during treatment. Average incidence is 2.8/10,000 person-years specifically for fibrate derivative. This increases to 5.9/10,000 for combined therapy of statins and fibrate.Citation[11] There is no case of rhabdomyolysis reported in the literature with only fenofibrate use; therefore, our patient is unique.
There is a wide variation in the clinical presentation of rhabdomyolysis. The classical triad of symptoms are muscle pain, weakness, and dark urine; however, these classical features are seen in fewer than 10% of the patients.Citation[1],Citation[7] Our patient also had muscle pain, weakness, and dark urine.
Although the excretion of fibrates is largely renal, they generally do not accumulate except in patients with renal failure.Citation[9],Citation[10] In a recent study, it was shown that 8–18% of fenofibrate-using patients with normal creatinine levels had a 5–8% rise in their creatinine levels.Citation[12] Our patient's creatinine level was elevated five times over the upper normal limit. Moreover, a rise in CPK level during therapy with antilipidemic drugs is well documented, ranging between 1–10% and usually not exceeding 10 times over the upper normal limit.Citation[9] Our patient's CPK level was elevated 300 times over the upper normal limit (see ).
The recommended daily dose for fenofibrate is 200–300 mg, which our patient had been prescribed. Myopathy usually appears within two weeks after the start of therapy; however, myopathy has been also reported after a long duration of treatment, such as two years.Citation[5],Citation[13] Similarly, symptoms of our patient became apparent only after a week of fenofibrate therapy.
As soon as rhabdomyolysis is recognized, attempts should be taken to prevent acute renal failure. Intravascular volume expansion through saline and sometimes mannitol to maintain urine output at more than 200–300 mL/ hour, with careful monitoring of sodium and calcium concentrations, is necessary. Early correction of hypovolemia reduces the incidence of renal failure. Alkalinization of urine prevents the dissociation of myoglobulin into globulin and ferrihemate, which are both toxic to the renal tubules. When renal failure ensues despite these measures, continuous hemofiltration or hemodialysis will be required.Citation[1],Citation[7],Citation[14] Saline, furosemide, and sodium bicarbonate infusions enabled diuresis in our patient, and this has led to a rapid recovery of her renal functions.
The prognosis depends not only on the underlying drug toxicity, which may contribute to the reported mortality of about 5–10% observed in serious rhabdomyolysis, but also in other accompanying systemic complications.Citation[1],Citation[8],Citation[15] However, with appropriate treatment, the outcome is generally good. In the rhabdomyolysis patients who need dialysis, the percentage of mortality has been found approximately as 40%.Citation[16] As our patient didn't require dialysis;, she recovered quickly and was discharged from the hospital totally recovered.
In summary, in spite of several cases of rhabdomyolysis associated with fibrate derivatives reported in the literature, few cases have been attributed to fenofibrate.Citation[17–20] In nearly all of the presented cases, there was a predisposing factor for rhabdomyolysis, such as diabetes, older age, hypothyroidism, or renal insufficiency. We couldn't demonstrate any other disorder other than hypertension in our patient.
Hence, physicians need to be aware of potentially fatal side effects such as rhabdomyolysis when prescribing fenofibrate. In addition, there should be a careful selection and monitoring of patients. Patient education about the risks and warning symptoms of potential adverse effects are necessary during or before the treatment. We recommend closely monitoring the symptoms of myopathy in patients treated with fenofibrate. If muscle weakness and/or elevations in the serum creatinine develop, rapid assessments of CPK and liver function tests may be helpful in the early diagnosis of renal impairment.
REFERENCES
- Vanholder R, Sever MS, Erek E, Lameire N. Rhabdomyolysis. J Am Soc Nephrol. 2000; 11: 1553–1561
- Ward MM. Factors predictive of acute renal failure in rhabdomyolysis. Arch Intern Med. 1988; 148: 1553–1557
- Staels B, Dallongeville J, Auwerx J, et al. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 1998; 98: 2088–2093
- Knoop RH. Drug treatment of lipid disorders. N Eng J Med. 1999; 341: 498–511
- Adkins JC, Faulds D. Micronised fenofibrate: A review of its pharmacodynamic properties and clinical efficacy in the management of dyslipidemia. Drugs. 1997; 54: 615–633
- Hodel C. Myopathy and rhabdomyolysis with lipid-lowering drugs. Toxicol Lett. 2002; 128: 159–168
- Zager RA. Rhabdomyolysis and myohemoglobinuric acute renal failure. Kidney Int. 1996; 19: 311–326
- Thomas MA, Ibels LS. Rhabdomyolysis and acute renal failure. Aust NZJ Med. 1985; 15: 623–630
- Dovignon J. Fibrates: A review of important issues and recent findings. Can J Cardiol. 1994; 10(S)61B–71B
- Miller DB, Spence JD. Clinical pharmacokinetics of fibric acid derivatives (fibrates). Clin Pharmocokinet. 1998; 14: 156–162
- Graham DJ, Staff JA, Shatin D, et al. Incidence of hospitalized rhabdomyolysis in patients treated with lipid–lowering drugs. JAMA. 2004; 292: 2585–2590
- Broeders N, Knoop C, Antoine M, Abramowicz D. Fibrate induced-renal dysfunction: Is gemfibrozil the only non-nephrotic agent?. Transplantation. 1999; 67S: S170
- Shepherd J. Fibrates and statins in the treatment of hyperlipidemia: An appraisal of their efficacy and safety. Eur Heart J. 1995; 16: 5–13
- Curry SC, Chong D, Connor D. Drug and toxin-induced rhabdomyolysis. Ann Emerg Med. 1989; 18: 1068–1084
- Gabow PA, Kaehny WD, Kelleher SP. The spectrum of rhabdomyolysis. Medicine. 1982; 61: 141–153
- Atef MR, Nadjatfi I, Boroumand B, Rastegar A. Acute renal failure in earthquake victims in Iran: Epidemiology and management. Q J Med. 1994; 87: 35–40
- Barker JB, Goodenough RR, Falko MJ. Fenofibrate monotherapy induced rhabdomyolysis. Diabetes Care. 2003; 26: 2482–2483
- Jones PH, Davidson MH. Reporting rate of rhabdomyolysis with fenofibrate + statin versus gemfibrozil + any statin. Am J Cardiol. 2005; 95: 120–122
- Gorriz JL, Sancho A, Lopez-Martin JM, et al. Rhabdomyolysis and acute renal failure associated with gemfibrozil monotherapy. Nephron. 1996; 74: 437–438
- Clouatre Y, Leblanc M, Ouimet D, Pichette V. Fenofibrate-induced rhabdomyolysis in two dialysis patients with hypothyroidism. Nephrol Dial Transplant. 1999; 14: 1047–1048