Abstract
The present study was performed to investigate whether the chronic administration of antioxidant vitamin C provided morphological protection on cisplatin-induced renal damage. Wistar albino male rats were divided into control and two experiment groups, each consisting of six rats. Cisplatin (5 mg/kg/month) was administered intravenously to the second and third group for three months. After the first application of cisplatin, vitamin C (8 mg/kg/day) to the third group was administered intramuscular for 3 months. At the end of the third month, the kidney specimens of the all groups were obtained. All of these kidney specimens were processed for light and electron microscopical examination. In the second group, most of the renal corpuscle lost their normal appearance and size, especially in the corticomedullary region. The most obvious changes were encountered in the proximal tubules. These changes were tubular dilation, thickening of basement membrane, loss of brush border, vacuolization, and swollenness of mitochondria in the proximal tubule epithelial cells. In addition, infiltration foci were observed mainly in the cortical region. In the third group, which was administered cisplatin plus vitamin C, although the structural damages and morphometric changes were lessened, mononuclear cell infiltration was still observed. This study suggests that the chronic administration of vitamin C may be of therapeutic benefit on cisplatin nephrotoxicity.
INTRODUCTION
Cisplatin (cis-diamminedichloroplatinum II) is one of the most potent chemotherapeutic antitumor agents. Activity has been demonstrated against a variety of neoplasms, particularly for head and neck, testicular, ovarian, bladder, and small cell lung cancers.Citation[1],Citation[2] High doses of cisplatin produce hepatoxicity, but the impairment of kidney function by cisplatin is recognized as the main side-effect and the most important dose-limiting factor.Citation[3–5] Cisplatin-induced renal damage is associated with increased renal vascular resistance and histologic damage to proximal tubular cells.Citation[1] The exact cellular mechanism of cisplatin-induced nephrotoxicity is insufficiently known.Citation[6],Citation[7] Thus, the effect of cisplatin has been studied in animals with the aim of understanding mechanisms of toxicity.Citation[5],Citation[8],Citation[9] The mechanism by which cisplatin induces renal injury is not well understood. It may involve direct interference with tubular or mitochondrial transport processes,Citation[10] the covalent modification of cellular constituents,Citation[11] or the generation of free radicals.Citation[12] Cisplatin-induced nephrotoxicity is closely associated with an increase in lipid peroxidation in the kidney tissues.Citation[1],Citation[5],Citation[13–16] This agent was able to generate reactive oxygen species (ROS), such as superoxide anions and hydroxyl radicals,Citation[3],Citation[4],Citation[17],Citation[18] and inhibit the activity of antioxidant enzymes in renal tissues.Citation[4],Citation[5],Citation[12] Cisplatin chemotherapy induces a fall in plasma antioxidant levels, which may reflect a failure of the antioxidant defense mechanism against oxidative damage induced by commonly used antitumor drugs.Citation[1],Citation[19] ROS may produce cellular injury and necrosis via several mechanisms, including the peroxidation of membrane lipids, protein denaturation, and DNA damage.Citation[20] Additionally, mitochondria are thought to be a major target of cisplatin, and mitochondrial DNA is heavily damaged by cisplatin,Citation[21] leading to mitochondrial loss of energy production and a release of a mitochondrial serin proteaseCitation[22] with subsequent cell death. Thus, protective agents have been developed to reduce the damage associated with antitumor drugs by providing site-specific protection for normal tissues without affecting the antitumor efficacy.Citation[1]
Vitamin C is an essential vitamin present in plant and animal cells.Citation[23] Vitamin C acts as a potent water-soluble antioxidant in biological fluidsCitation[24] by scavenging physiologically relevant ROS and reactive nitrogen species.Citation[25] Thus, it may thereby prevent oxidative damage to important biological macromolecules such as DNA, lipids, and proteins.Citation[26] In addition, it can regenerate the reduced form of α-tocopherol, perhaps accounting for observed sparing effects of these vitamins.Citation[27] However, few papers have reported on the effects of this vitamin in cisplatin treated rats, and usually only a single dose has been used. The present study was performed to investigate possible protective effects of treatment with chronic administration of antioxidant vitamin C on cisplatin nephrotoxicity in Wistar rats.
MATERIALS AND METHODS
Chemicals
Cisplatin (Cisplatin DBL®, Orna Chemicals and Pharmaceutics), vitamin C (Redoxon®, Roche Chemicals and Pharmaceutics) and dexamethasone (Dekort®, Deva Chemicals and Pharmaceutics) were purchased on the local market. Vitamin C was diluted in distilled water as a concentration consisting of 0.32 mg/kg per ml. All other chemicals and reagents used were of analytical grade.
Animals
Eighteen Wistar albino male rats (three months old, weighing 250–300 g) were used in this study. They were fed daily tap water and pellet foods including 21% pure protein under optimum laboratory conditions (temperature, 21°C; humidity, 40–60%; light/dark period: 12h/12h, optimum air condition system).
Experimental Design
The animals were divided into two treatment and control groups of six rats each (n = 6). Control rats were daily injected intramuscular (i.m.) serum physiologic for three months. Cisplatin-treated rats (group II) were repeatedly injected intravenously (i.v.) three times at 21 day intervals with cisplatin (5 mg/kg). Cisplatin + vitamin C-treated rats (group III) were injected cisplatin (i.v., 3 times at 21 day intervals, 5 mg/kg) and additionally vitamin C (i.m., 8 mg/kg) daily following the first injection of cisplatin for three months. Groups II and III rats pre-treated dexamethasone (0.13 mg/kg), i.m., twice (interval: 6 h), 24 h before cisplatin i.v. injection against several allergic reactions, possible to come out, due to cisplatin chemotherapy.
Histopathological Procedures
At the end of the experiments, the animals of each group were killed by decapitation. The kidney specimens of the all groups were obtained and then processed for light and electron microscopical examination.
For light microscopical observation, kidney specimens were embedded in the paraffin blocks after they had been fixed in Bouin's solution. Five micrometer (μm) sections were obtained and stained with hematoxylin + eosin, periodic acid-Schiff+hemalen, and Masson's trichrome.
For electron microscopical observation, kidney parts in 1 × 2 mm size were fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer, and after primary fixations tissues were washed in 0.1 M phosphate buffer overnight. The tissues were postfixed with 1% osmium tetroxide in phosphate buffer for 1 h at 4°C. Then the postfixed tissues were washed in 0.1 M phosphate buffer and dehydrated by graded ethyl alcohol and finally with propylene oxide. Dehydrated tissues were processed for making araldite blocks. Ultrathin sections were obtained by ultramicrotome (RMC-MTX Ultramicrotome-USA) and collected on copper grids for double staining (uranyl acetate and Reynold's lead citrate). Stained sections were finally observed under a Jeol-JEM 1010 transmission electron microscope.
Morphometric Analysis
Five micrometer paraffin sections were stained with hematoxylin+eosin for morphometry. Twenty glomeruli and tubules from each rat (i.e., 120 glomeruli and tubules for each group) were chosen randomly. The randomly selected different glomeruli (at a magnification of 20 ×) and tubules (at a magnification of 40 ×) in the cortex of section were evaluated. The diameters of each glomeruli and tubules were measured under a light microscope (Olympus Cx 31) using a micrometric ocular. Two different diameters (i.e., the greatest and the smallest) of each glomeruli were measured. The average diameters of glomeruli were calculated.
Statistical Analysis
The data were expressed as mean ± standard deviation (SD). Normality distribution of the variables was tested using one sample Kolmogorov-Smimov test. Differences in measured parameters among the groups were analyzed with a nonparametric Kruskal-Wallis test due to non-normal distribution. When a significance difference was found, Bonferroni post-hoc test was used for multiple comparisons. A p value < 0.05 was considered statistically significance. Statistica 7.0 software was used for all statistical analyses.
RESULTS
When the kidney specimens obtained from the control group were examined as light and electron microscopic, normal histological structure of the glomeruli and renal tubules were observed (see , , , and ).
Figure 1. Histological examination of rat kidney, from the regions of cortex. (A, B) Control rat kidney, showing normal morphology. (C, D) Cisplatin-treated rat kidney, showing massive dilation of tubules (asterisk), tubular necrosis (arrowhead), cast formation in the lumen (arrowhead-ball), and focal mononuclear cells infiltration (arrow). (E, F) Cisplatin + vitamin C-treated rat kidney, showing slight dilation of tubules and degenerative changes (hematoxylin + eosin, scale bar: 100 μm).
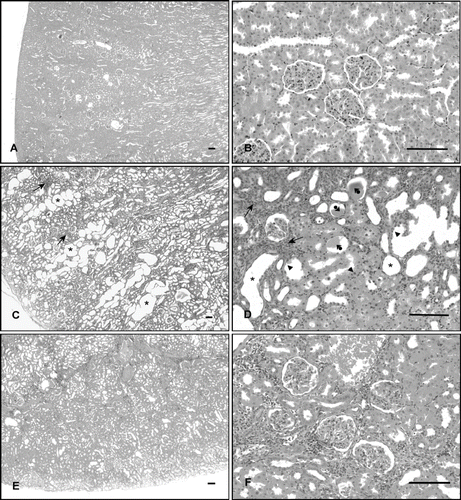
Figure 2. Photomicrographs of kidney sections stained with hematoxylin-eosin (A, D, G), Masson's trichrome (B, E, H), and periodic acid-Schiff-hemalen (C, F, I). (A, B, C) Control rats. (D, E, F) Cisplatin-treated rats, arrowhead; tubular epithelial cell exhibited enlarged nuclei with basophilic cytoplasm, arrow; thickening of the basement membrane in the renal tubules and Bowman's capsule, asterisk; enlarged periglomerular spaces. (G, H, I) Cisplatin + vitamin C-treated rats (Scale bar: 50 μm).
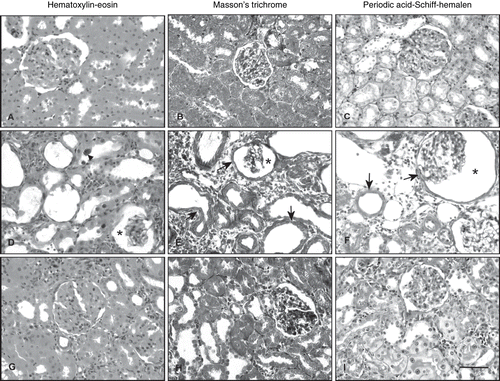
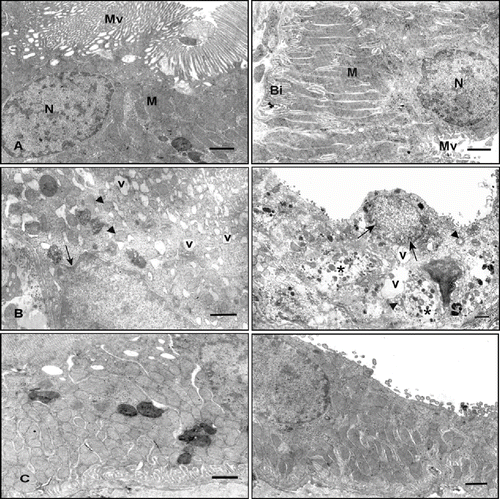
Figure 3. Electron micrographs of rat renal tubules. Left panel shows proximal renal tubules, right panel shows distal tubules. (A) Control rat. Left and right panels show normal morphology. (B) Cisplatin-treated rat. Left and right panels show numerous cytoplasmic vacuoles (v), invagination of the nuclear envelope (arrow), rounded mitochondria with disordered cristae (arrowhead), shortening and loss of basal infoldings, partial loss of cytoplasm (asterisk), and microvilli. (C) Cisplatin + vitamin C-treated rat. Left and right panels show little evidence of damaged cellular structure (uranyl acetate and lead citrate, scale bar: 1μm). Abbreviations: microvilli (Mv), nucleus (N), mitochondria (M), and basal infoldings (Bi).
In the light microscopic examinations of cortical region of the kidney 12 weeks after repeated i.v. injections of cisplatin (5 mg/kg, 3 times at 21 day intervals), the most characteristic feature was massive tubular dilation. These dilations extended to involve both the inner and outer cortices (see ), flattened the epithelial lining of the dilated tubules, and cast formation in some of the tubular lumens (see ). Occasionally, focal tubular necrosis was seen, and sloughed cells were found in some tubular lumens (see ). Some tubular epithelial cell exhibited enlarged nuclei with basophilic cytoplasm, suggesting an increased cell metabolism (see ). However, there was no sign of active tubular regeneration. Some glomeruli seemed to have disappeared, and the remaining glomeruli often showed enlarged periglomerular spaces (see ). The tickening of the basement membrane in the renal tubules and Bowman's capsule was also seen (see and ). There was markedly focal mononuclear cell infiltration, mainly in the cortex. Mononuclear cell infiltration was also evident among some peritubular and periglomerular areas (see and ).
Electron microscopic examinations of the renal tubular cells showed numerous cytoplasmic vacuoles, rounded mitochondria with disordered cristae, shortening and loss of basal infoldings, partial loss of cytoplasm and microvilli, and invagination of the nuclear envelope (see ).
Treatment of rats with vitamin C ameliorated the pathological changes induces by cisplatin (see and ). The severity of degenerative changes in the glomerules and especially tubules of renal cortex were less than those in the cisplatin group (see ). Although epithelial cell degenerations and tubular dilation decreased, mononuclear cell infiltration was still observed (see ).
An ultrastructural examination of the renal tubular cells in the vitamin-treated groups revealed little evidence of damaged cellular structure (see ).
The diameter of glomerules and tubules in the renal tissues was measured and was calculated according to these results (see ). Although cisplatin significantly decreased the diameter of glomerules (p < 0.01), it increased the diameter of renal tubules (p < 0.01) compared to that of the control. Vitamin C treatment significantly increased the diameter of glomerules (p < 0.05) and decreased the diameter of renal tubules (p < 0.05) compared to cisplatin group.
Table 1 Comparison diameter of glomerules and tubules in renal tissues
DISCUSSION
Cisplatin is an effective chemotherapeutic agent used in the treatment of a wide variety of solid tumors.Citation[5],Citation[28] The major side effect limiting its clinical use is dose-related nephrotoxicity.Citation[4],Citation[5],Citation[28],Citation[29] The current package circular issued by its manufacturer warns that renal toxicity may occur in 28–36% of patients receiving a single dose of cisplatin of 50 mg/m2.Citation[2]
Cisplatin is mainly excreted from the kidney, and the kidney tissue content of this agent is higher than the concentrations in other organs. As cisplatin is retained in the kidney tissue for a long duration, it may readily cause nephrotoxicity. It has also been reported that cisplatin-related nephrotoxicity develops in a dose-dependent manner in animals and humans.Citation[30] In animal models, the S3 segment of the proximal tubule appears to be major site of renal injury.Citation[2],Citation[18],Citation[29],Citation[31] In humans, however, damage seems to occur primarily in the distal tubule and collecting ducts.Citation[2],Citation[4],Citation[32],Citation[33]
The present study shows that repeated injection of cisplatin (5 mg/kg, i.v., three times at 21-day intervals) induced histopathological and morphometric changes in rat kidney that were reversed to a considerable extent by the chronic administration of vitamin C (8 mg/kg, i.m., daily following the first injection of cisplatin for three months). Cisplatin treatment induced massive tubular dilation and cast formation in some of the renal tubular lumens. Occasionally, focal tubular necrosis, a thickening of the basement membrane in the renal tubules and Bowman's capsule, and focal mononuclear cell infiltration were seen. Renal tubular damage was present with vacuolization and desquamation of epithelial cells. Some glomeruli seemed to have disappeared, and the remaining glomeruli often showed enlarged periglomerular spaces. These data are corroborated by previous studies reported by other investigators on cisplatin-induced nephrotoxicity in rats.Citation[9],Citation[20],Citation[34],Citation[35]
Cisplatin-induced nephrotoxicity has been shown to be dose-related in both animals and humans. Prolonged weekly injections in rats cause tubular atrophy of cortical nephrons, cystic dilation of inner cortical or medullary tubules, and chronic renal failure due to tubulointerstitial nephritis.Citation[32] Earlier studies indicate that cisplatin exerts its effect on the S3 segment of the proximal tubule located that in the outer stripe of the medulla.Citation[36],Citation[37] Choie et al. have shown the platinum concentration in the kidney is the highest in the corticomedullary region and the least in medulla. When compared with the histologic observations, the most severe tubular lesions localized in the corticomedullary region corresponded with the highest tissue concentration of platinum in that region.Citation[34]
The kidney receives a high exposure to cisplatin as the major route of cisplatin elimination via urine by filtration at the glomeruli and secretion in the proximal convoluted tubules.Citation[4] Choie et al. have shown that chronic cisplatin treatment induced glomerular changes in rat kidney similar to our results. Citation[34]
In our study, we also observed markedly focal mononuclear cell infiltration, mainly in the cortex. These data are corroborated by previous studies.Citation[7],Citation[9],Citation[19],Citation[28] Blisard et al. noted that the cells in the interstitial tissue are both mononuclear and polymorphonuclear cells,Citation[8] while el-Shazly et al.Citation[9] and Guinee et al.Citation[28] observed that these cells are lymphocytes.
Numerous studies in experimental animals treated with nephrotoxic heavy metals in vivo have described shortening and focal loss of microvilli in the proximal tubule cells, a phenomenon that may partially explain a well-known impairment of reabsorptive and secretory functions of the proximal tubule cells. As the cell membrane represents the first organelle exposed to the heavy metal after being filtered in the glomerulus, the well-known nephrotoxic heavy metals could directly bind to brush border membrane and damage its integrity. This may increase the permeability of the membrane and cause the loss of microvilli. An interaction of heavy metals with the proximal tubule cell basolateral membrane may lead to fragmentation and loss of basolateral invaginations. Our electron microscopic observations were in agreement with those in the literature: tubular changes such as shortening and loss of basal infolding, rounded mitochondria with disordered cristae, focal loss of brush border, and invagination of the nuclear envelope.Citation[15],Citation[31],Citation[34],Citation[38]
Dose-limiting toxicity of the antitumor agents is mainly attributed to the inability of these drugs to differentiate between normal and tumour cells. Protective agents have been developed to reduce the damage associated with antitumor drugs by providing site-specific protection for normal tissues without affecting the antitumor efficacy.Citation[1] Enzymatic and molecular defence mechanisms are present in the cell to prevent the integrity of biological membranes from oxidative processes caused by free radicals. The administration of antioxidants such as vitamin E, vitamin C, lycopene, and melatonin, before or after treatment with cisplatin, has been used to protect or ameliorate against nephrotoxicity in human and animals.Citation[5],Citation[6],Citation[20],Citation[39–41]
Much attention has been given to the possible role that dietary antioxidants play in protecting against cisplatin-induced nephrotoxicity.Citation[6],Citation[42] Vitamin C is a water-soluble dietary antioxidant that plays an important role in controlling the oxidative stress.Citation[43] This vitamin, as an antioxidant agent, may have inhibited the chain reactions of the cisplatin-generated free radicals or scavenged the free radicals before they reached the cell targets damaging the glomerular kidney functions.Citation[3] It can also protect DNA against damages induced by various chemicals.Citation[44] It has been previously shown that vitamin C successfully reduced the clastogenic effect of many antitumor agents and radiation in vivo assays. Vitamin C also has in vivo anticlastogenic effects against chromosomal damage induced by cisplatin in rodents.Citation[45] However, few papers have reported on the effects of this vitamin in cisplatin-treated rats, and usually single dose has been used.
Antunes et al. have reported that vitamin C showed protection in a dose-dependent manner on cisplatin-induced oxidative damage on adult Wistar rat kidneys.Citation[3] Gemba et al. have shown that mitochondria in the cultured cells treated with cisplatin were fewer than in the control cells and had swollen; these change caused by cisplatin tended to be smaller when vitamin C was present.Citation[39]
Cisplatin cross-links to DNA, forming intra- and interstrand adducts that bend and unwind the duplex and attract high-mobility-group domain and other proteins. Presumably due to a shielding effect caused by these proteins, the cisplatin-modified DNA is poorly repaired. The resulting DNA damage triggers cell-cycle arrest and apoptosis.Citation[46] In animals, vitamin C inhibited the development of estrogen-induced kidney tumors in hamsters by blocking the formation of DNA adducts.Citation[47] Vissers et al. reported that intracellular ascorbate can specifically protect apoptosis in human umbilical vein endothelial cells (HUVEC) in the presence of chlorinated oxidants.Citation[48]
In the present study, a significant decrease in the severity of histopathological and morphometric changes induced by cisplatin was observed in rat kidney treated with vitamin C plus cisplatin when compared with the cisplatin group.
In conclusion, chronic administration of vitamin C would be effective in protecting against cisplatin-induced tissue damage in rat kidneys. It is possible that the toxic effect of cisplatin is some how minimized by a compensatory mechanism involving vitamin C via the induction of antioxidant enzyme activity following intravenous injection of cisplatin.
ACKNOWLEDGMENT
This study was supported as Project 444 by Trakya University Research Center, Edirne, Turkey.
REFERENCES
- Antunes LM, Darin JD, Bianchi MD. Effects of the antioxidants curcumin or selenium on cisplatin-induced nephrotoxicity and lipid peroxidation in rats. Pharmacol. Res. 2001; 43: 145–150
- Meyer KB, Madias NE. Cisplatin nephrotoxicity. Miner. Electrolyte. Metab. 1994; 20: 201–213
- Antunes LM, Darin JD, Bianchi MD. Protective effects of vitamin C against cisplatin-induced nephrotoxicity and lipid peroxidation in adult rats: A dose-dependent study. Pharmacol. Res. 2000; 41: 405–411
- Vickers AEM, Rose K, Fisher R, Saulnier M, Sahota P, Bentley P. Kidney slices of human and rat to charactarize cisplatin-induced injury on cellular pathways and morphology. Toxicol Pathol. 2004; 32: 577–590
- Durak I, Ozbek H, Karaayvaz M, Ozturk HS. Cisplatin induced acute renal failure by impairing antioxidant system in guinea pigs: Effects of antioxidant supplementation on the cisplatin nephrotoxicity. Drug Chem Toxicol. 2002; 25: 1–8
- Appenroth D, Frob S, Kertsen L, Splinter FK, Winnefeld K. Protective effects of vitamin E and C on cisplatin nephrotoxicity in developing rats. Arch. Toxicol. 1997; 71: 677–683
- Saleh S, el-Demerdash E. Protective effects of L-arginine against cisplatin-induced renal oxidative stress and toxicity: Role of nitric oxide. Basic Clin Pharmacol Toxicol. 2005; 97: 91–97
- Blisard KS, Harrington DA. Toxicity of cis-diamminedichloroplatinum (II) (Cisplatin) in the frog. Rana pipiens. J. Comp. Pathol. 1990; 103: 387–398
- el-Shazly MO, Afify MM, el-Dieb MK. Histopathological study into side-effect toxicity of some drugs used in treatment of cancer. Arch Exp Veterinarmed. 1989; 43: 319–326
- Zhang J, Lindup E. Cisplatin nephrotoxicity: Decrease in mitochondrial protein sulphydryl concentration and calcium uptake by mitochondria from rat renal cortical slices. Biochem. Pharmacol. 1994; 47: 1127–1135
- Mistry P, Merazga Y, Spargo DJ, Riley PA, McBbrien DCH. The effects of cisplatin on the concentration of protein thiols and glutathione in the rat kidney. Cancer Chemother Pharmacol. 1991; 28: 277–282
- Sadzuka Y, Shoji T, Takino Y. Effect of cisplatin on the activities of enzymes which protect against lipid peroxidation. Biochem. Pharmacol. 1992; 43: 1872–1875
- Hannemann J, Baumann K. Cisplatin-induced lipid peroxidation and decrease of gluconeogenesis in rat kidney cortex: Different effects of antioxidants and radical scavengers. Toxicology. 1988; 51: 119–132
- Sadzuka Y, Shoji T, Takino Y. Mechanism of the increase in lipid peroxide induced by cisplatin in the kidneys of rats. Toxicol. Lett. 1992; 62: 293–300
- Sugihara K, Nakano S, Koda M, Tanaka K, Fukuishi N, Gemba M. Stimulatory effect of cisplatin on production of lipid peroxidation in renal tissues. Jpn J Pharmacol. 1987; 43: 247–252
- Fukushi N, Gemba M. Use of cultured renal epithelial cells for the study of cisplatin toxicity. Jpn J Pharmacol. 1989; 50: 247–249
- Nishikawa M, Nagatomi H, Nishijima M, et al. Targeting superoxide dismutase to renal proximal tubule cells inhibits nephrotoxicity of cisplatin and increases the survival of cancer-bearing mice. Cancer Lett. 2001; 171: 133–138
- Sueishi K, Mishima K, Makino K, et al. Protection by a radical scavenger edaravone against cisplatin-induced nephrotoxicity in rats. Eur. J. Pharmacol. 2002; 451: 203–208
- Bompart G, Orfila C. Cisplatin nephrotoxicity in lead-pretreated rats: Enzymatic and morphological studies. Toxicol Lett. 1990; 50: 237–247
- Atessahin A, Yilmaz S, Karahan I, Ceribasi AO, Karaoglu A. Effects of lycopene againts cisplatin-induced nephrotoxicity and oxitadive stress in rats. Toxicology. 2005; 212: 116–123
- Olivero OA, Semino C, Kassim A, Lopez-Larraza DM, Pirier MC. Preferential binding of cisplatin to mitochondrial DNA of chinese hamster ovary cells. Mutat. Res. 1995; 346: 221–230
- Cilenti L, Kyriazis GA, Soundarapandian MM, et al. Omi/HtrA2 protease mediates cisplatin-induced cell death in renal cells. Am. J. Physiol. Renal Physiol. 2005; 288: 371–379
- Tsao CS. An overview of ascorbic acid chemistry and biochemistry. Vitamin C in Health and Disease., L Packer, J Fuchs. Marcel Dekker, New York 1997; 25–58
- Frei B, Stocker R, England L, Ames BN. Ascorbate: The most effective antioxidant in human blood plasma. Adv. Exp. Med Biol. 1990; 264: 155–163
- Halliwell B, C vitamin. Antioxidant or pro-oxidant in vivo?. Free Radical Res. 1996; 25: 439–454
- Carr A, Frei B. Does vitamin C act as a pro-oxidant under physiological conditions?. FASEB J. 1999; 13: 1007–1023
- Padh H. Vitamin C: Newer insight into its biochemical functions. Nutr Rev. 1991; 49: 65–70
- Guinee DG, van Zee B, Houghton DC. Clinically silent progressive renal tubulointerstitial disease during cisplatin chemotherapy. Cancer. 1993; 71: 4050–4054
- Brady HR, Kone BC, Stromski ME, Zeidel ML, Giebisch G, Gullans SR. Mitochondrial injury: An early event in cisplatin toxicity to renal proximal tubules. Am J Physiol. 1990; 258: 1181–1187
- Kawai Y, Taniuchi S, Okahara S, Nakamura M, Gemba M. Relationship between cisplatin or nedaplatin-induced nephrotoxicity and renal accumulation. Biol. Pharm. Bull. 2005; 28: 1385–1388
- Orfila C, Bompart G, Lepert JC, Suc JM, Girolami JP. Renal immunolocalization of kallikrein in cisplatin nephrotoxicity in rats. Histochem J. 1993; 25: 772–777
- Daugaard G, Abildgaard U. Cisplatin nephrotoxicity. Cancer Cemother. Pharmacol. 1989; 25: 1–9
- Kanaka C, Oetliker OH, Bianchetti MG. Chronic cisplatin tubulopahty in humans and animals: Clear-cut discrepant findings. Nephron. 1991; 59: 693
- Choie DD, Longnecker DS, del Campo AA. Acute and chronic nephropathy in rats. Lab. Invest. 1981; 44: 397–402
- Mohan IK, Khan M, Shobha JC, et al. Protection against cisplatin-induced nephrotoxicity by spirulina in rats. Cancer Chemother. Pharmacol. 2006; 58: 802–808
- Dobyan DC, Levi J, Jacobs C, Kosek J, Weiner NW. Mechanism of cisplatinum nephrotoxicity II: Morphologic observations. J. Pharmacol. Exp. Ther. 1980; 7: 551–556
- Racusen LC, Solez R. Nephrotoxic tubular and interstitial lesions: Morphology and classification. Toxicol Pathol. 1986; 14: 45–49
- Herak-Kramberger CM, Sabolic I. The integrity of renal cortical brush-border and basolateral membrane vesicles is damaged in vitro by nephrotoxic heavy metals. Toxicology. 2001; 156: 139–147
- Gemba M, Fukuishi N. Amelioration by ascorbic acid of cisplatin-induced injury in cultured renal. Contrib Nephrol. 1991; 95: 138–142
- Sener G, Satiroglu H, Kabasakal L, et al. The protective effect of melatonin on cisplatin nephrotoxicity. Fundam Clin Pharmacol. 2000; 14: 553–560
- Naziroglu M, Karaoglu A, Aksoy AO. Selenium and high dose vitamin E administration protects cisplatin-induced oxidative damage to renal, liver and lens tissues in rats. Toxicology. 2004; 195: 221–230
- Bogin E, Marom M, Levi Y. Changes in serum, liver and kidneys of cisplatin-trated rats: Effects of antioxidants. Eur J Clin Chem Clin Biochem. 1994; 32: 843–851
- Panayiotidis M, Collins AR. Ex vivo assesment of lymphocyte antioxidant status using the commet assay. Free Radical Res. 1997; 27: 533–537
- Blasiak J, Kowalik J. Protective action of vitamin C against DNA damage induced by selenium-cisplatin conjugate. Acta Biochim Pol. 2001; 48: 233–240
- Antunes LMG, Araújo MCP, Darin JDC, Bianchi MLP. Effects of the antioxidants curcumin and vitamin C on cisplatin-induced clastogenesis in Wistar rat bone marrow cells. Mutat. Res. 2000; 465: 131–137
- Jordan P, Carmo-Fonseca M. Molecular mechanisms involved in cisplatin cytotoxicity. Cell Mol Life Sci. 2000; 57: 1229–1235
- Block G, Henson DE, Levine M, vitamin C. A new look. Ann Intern Med. 1991; 114: 909–910
- Vissers MCM, Lee WG, Hampton MB. Regulation of apoptosis by vitamin C. J. Biol. Chem. 2001; 276: 46835–46840