Abstract
Despite proven renoprotection from RAAS blockade and its increased application since the early 1990s, we have experienced an increasing CKD/ESRD epidemic, especially among U.S. diabetics. Consequently, some concerns regarding iatrogenic azotemia from RAAS blockade have surfaced. We hypothesized that susceptible CKD patients with normal renal arteries on conventional angiography, including MRA, but who have microvascular arteriolar narrowing in the renal circulation—mimicking large vessel renal artery stenosis, even without precipitating risk factors—could experience worsening azotemia after periods of time exceeding three months on stable doses of RAAS blockade. Between September 2002 and February 2005, as part of a larger prospective study of renal failure in CKD patients on RAAS blockade, we studied five patients with >25% higher serum creatinine and normal MRA without precipitating factors. RAAS blockade was discontinued. eGFR by MDRD was monitored. Five Caucasians (M:F = 1:4; age 68 years) were enrolled and followed-up at 29.6 months. The duration of RAAS blockade at enrollment was 34.6 months. The baseline eGFR had decreased from 28.4 ± 7.1 to 17.0 ± 7.4 ml/min/1.73 m2 BSA (p < 0.001) at enrollment. One required temporary hemodialysis; no deaths occurred. eGFR increased from 17.0 ± 7.4 to 24.6 ± 9.5 ml/min/1.73 m2 BSA (p = 0.009), 29.6 (20–43) months after stopping the RAAS blockade. We conclude that worsening azotemia occurs in susceptible CKD patients on stable doses of RAAS blockade after long periods of time, despite normal renal arteries without precipitating risk factors. We submit that microvascular renal arteriolar narrowing is the pathophysiologic mechanism. These observations call for further study.
INTRODUCTION
Since the mid 1990s, an evidence-based consensus has emerged of enhanced cardio/renoprotection by RAAS blockade with ACEIs, ARBs, and/or aldosterone antagonists.Citation[1–15] Several large multi-center randomized placebo controlled trials have shown renoprotection with RAAS blockade, beyond blood pressure lowering, in CKD patients with diabetic nephropathyCitation[3],Citation[7],Citation[9–12] and in non-diabetic CKD patients with hypertension.Citation[4] As a result, the last two decades have witnessed an escalating use of RAAS blocking agents in clinical medicine.Citation[16–19] Nearly 80% of U.S. diabetic patients are receiving an ACE inhibitor, an ARB, or a combination of both agents.Citation[17],Citation[19]
Despite this widening application of RAAS blocking strategies in the United States, during the past two decades, the incidence of ESRD/CKD, especially among U.S. diabetics, had continued to rise, a rate of increase that had outpaced the rate of increase of the diabetes epidemic.Citation[20–22] Such observations and trends have led to increasing concerns regarding iatrogenic ESRD and CKD associated with the use of ACE inhibitors and ARBs in general medical practice.Citation[20],Citation[21] Furthermore, in a recent population-based historical cohort analysis of 6102 diabetic patients in Canada (mean age 66 years), Suissa et al. demonstrated an increased rate ratio of ESRD of 4.2 (95% CI: 2.0–9.0), after three years or longer, of ACE inhibition.Citation[23]
The association of accelerated renal failure in CKD patients concurrently receiving ACE inhibitors, ARBs, or a combination of both is well reported and acknowledged in the medical literature.Citation[24–37] Precipitating risk factors reported include initiation of RAAS blockade, volume depletion, hypotension and cardiovascular hemodynamic instability, the presence of hemodynamically significant renal artery stenosis, use or abuse of NSAIDs or cox II inhibitors with or without concurrent administration of diuretics, the presence of severe illness or infections following a dose increase or switch between an ACE inhibitor and an ARB, and exacerbation of heart failure.Citation[24–37]
Against this backdrop, and cognizant of anecdotal observations made by the first author about ten years ago in a practice in Baltimore, Maryland, we hypothesized that susceptible CKD patients with normal renal arteries on conventional angiography, including MRA, but who have microvascular arteriolar narrowing in the renal circulation, mimicking large vessel renal artery stenosis, could experience worsening azotemia after long periods of time (>3 months), on stable doses of RAAS blockade, even without precipitating risk factors. We surmised that this syndrome may be under-recognized and therefore required further prospective examination. The rationale of our study was to identify CKD patients
with normal renal arteries on MRA,
who have been on the same dose of RAAS blocking agent or agents for periods >3 months, and
who present with worsening symptomatic azotemia,
and to follow eGFR after stopping the ACE inhibitor, ARB, or combination of an ACE inhibitor and an ARB. It must be acknowledged that previous reports have demonstrated only small increases in GFR, usually less than 6%, one month following withdrawal of chronic antihypertensive therapy.Citation[38],Citation[39] Remarkably, in both studies, there were no demonstrable differences in the observed increases in GFR between patients previously on ACE inhibitor compared to patients previously on a beta blocker.Citation[38],Citation[39]
PATIENTS AND METHODS
Between September 2002 and February 2005, in a Mayo Health System Hypertension Clinic at the Midelfort Clinic, Eau Claire, Wisconsin, we prospectively enrolled all CKD patients on an ACE inhibitor and/or an ARB who developed worsening azotemia with >25% increase in baseline serum creatinine, usually in the preceding 3 months or less. Standard nephrology care was administered with diagnosis-directed laboratory tests, including urinalysis and culture, renal sonogram, serology testing, microbiology, and toxicology. MRA was obtained only as a function of medical need or necessity and was not available in all patients. RAAS blockade was discontinued. As a subgroup, we will in this report analyze the renal and patient outcomes in the patients with normal renal arteries (on MRA) on the same dose of an ACE inhibitor, an ARB, or a combination of both for three months or longer, and without precipitating risk factors. Patients with abnormal renal arteries on MRA, patients with precipitating risk factors, patients with less than three months of stable RAAS blockade prior to enrollment, and patients lost to follow-up or with a follow-up of less than three months were excluded from this analysis. Kidney function, as measured by serum creatinine and eGFR by MDRD formula,Citation[40] was monitored every one or two weeks for the first month, monthly for three months, and then every two or three months thereafter. Serum creatinine values for each individual patient over time were collected, analyzed, and expressed as MDRD-estimated GFR in ml/minute/1.73 m2 BSA.Citation[40] We used straight-line graphs to depict changes of eGFR in selected patients. Data were also shown in tables. All available EMR and chart-based data, locally, statewide, and beyond, were accessed for follow up, where possible.
For all continuous variables, the results are reported as means ± SD, with ranges shown in parenthesis. Differences between two means were calculated using the Student's t test method, and a p value of <0.05 was considered to be statistically significant. Paired t test was used to compare differences within groups following an intervention whereas unpaired t test was used to compare differences between groups.
RESULTS
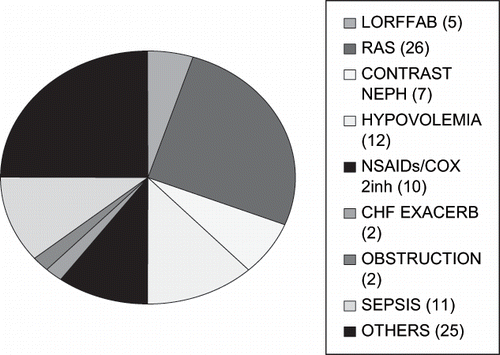
As a cohort, 100 Caucasian CKD patients were enrolled during the 30-month period, September 2002 through February 2005. We completed a 50-month follow-up in November 2006. There were 52 males, 48 females, mean age 71.5 (25–92) years. Associated precipitating risk factors for renal failure were present in 95 of 100 (95%) patients, including contrast-induced nephropathy,Citation[7] hypovolemia,Citation[12] hemodynamically significant RAS,Citation[26] use/abuse of NSAIDs/cox II inhibitors,Citation[10] heart failure,Citation[2] sepsis,Citation[11] and 25 others had miscellaneous risk factors, including post-operative states, malignancies, post-chemotherapy, acute myocardial infarction, and hypercalcemia (see ). Patient and renal outcomes in the remaining 5 of 100 (5%) patients will follow.
Figure 1. Spectrum of associated precipitating risk factors in 100 CKD patients at enrollment. Abbreviations: RAS = renal artery stenosis, LORFFAB = Late onset renal failure from angiotensin blockade.
Five patients, one male and four females with a mean age 68 years (44–83), were eligible to enter the analysis. Three were diabetic hypertensives, one hypertension alone, and one hypertension with stable SLE. They were on multiple antihypertensives, 2–5 each at presentation, as shown in . Mean duration of RAAS blockade before presentation was 34.6 (17–60) months. Lisinopril was common, used by 4/5 patients, mean dose of 21 (5–40) mg/day. RAAS blockade was discontinued in 4 patients, and the dose of the ARB, candesartan, was halved in the fifth. Three patients received alternate antihypertensive agents (see ). Blood pressure changes following discontinuation of RAAS blockade are shown in : MABP at enrollment into the study was 94.6 ± 5.5 (92–104) mm Hg and compared to MABP values at last clinic follow-up of 101.4 ± 10.1 (89–111) mm Hg (p, NS).
Table 1 Profile of antihypertensive treatment in 5 patients with LORFFAB
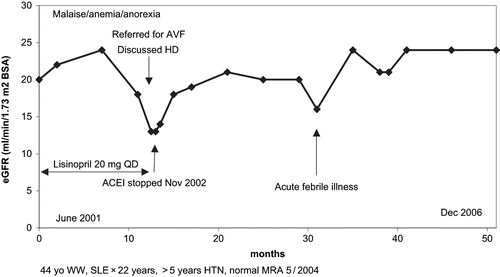
The mean baseline serum creatinine in all five patients prior to presentation was 2.1 ± 0.5 (1.4–2.9) mg/dL. This compared to a lower presentation mean serum creatinine of 3.4 ± 1.1 (1.8–4.6) mg/dL, p = 0.01. Similarly, mean baseline eGFR prior to presentation in all five patients was 28.4 ± 7.1 ml/min/1.73 m2 BSA compared to a lower enrollment mean eGFR value of 17.0 ± 7.4 (12–30) ml/min/1.73 m2 BSA, (p < 0.001). Mean serum creatinine decreased from 3.4 ± 1.1 (1.8–4.6) mg/dL to 2.1 ± 0.6 (1.4–3.0) mg/dL, 29.6 (20–43) months after withdrawal of RAAS blockade, (p = 0.009). Correspondingly, following discontinuation of RAAS blockade, mean eGFR increased from 17.0 ± 7.4 (12–30) ml/min/1.73 m2 BSA to 24.6 ± 9.5 (17–41) ml/min/1.73 m2 BSA, p = 0.008. Urinary albumin creatinine ratio increased following withdrawal of RAAS blockade. Patient 1 required HD for 10.5 months, discontinued HD in February 2005, and remains dialysis-independent with a serum creatinine of 3.0 mg/dL, 21 months later, as at November 2006. Twenty-four-hour urine creatinine clearance measurements, available in 4/5 patients, showed good correlation with MDRD eGFR estimations, R = 0.93. Four of five (80%) patients developed anemia, and all five demonstrated secondary hyperparathyroidism; both indices improved as kidney function got better for all patients. Changes in eGFR in patient 5 are represented graphically in . Patient 5, a 44-year-old Caucasian woman who was known hypertensive with stable systemic lupus erythematosus, was referred to our unit in November 2002 with symptomatically worsening renal failure, baseline serum creatinine increasing from 2.3 mg/dL to 4.3 mg/dL, after 17 months of ACE inhibition with lisinopril 20 mg/day, to start hemodialysis and for arteriovenous fistula placement. There was no clinical or serologic evidence for a lupus flare up. Presenting symptoms included generalized weakness, easy fatigability, and anorexia. She was anemic. MRA for the renal arteries was normal. Lisinopril was withdrawn, and within weeks, serum creatinine had decreased to below 3.0 mg/dL with eGFR increasing from presentation values of about 10 ml/min/1.73 m2 BSA to nearly 20 ml/min/1.73 m2 BSA (see ). In November 2006, 48 months after discontinuation of lisinopril, patient's eGFR remains stable at 25 ml/min/1.73 m2 BSA (see ), and patient remains strong, with excellent appetite. She is continuing on maintenance erythropoietin and works as a part-time secretary in a local office. She never started hemodialysis, and so far, we have not requested for a surgery consult for an arteriovenous fistula placement.
DISCUSSION
In this prospective study, we have described a new syndrome, previously poorly recognized, of late onset renal failure from angiotensin blockade (LORFFAB), occurring in CKD patients with normal renal arteries, on the same dose of RAAS blocking therapy for >3 months to several years, and without any identifiable precipitating risk factors. Our preliminary results were published previously.Citation[41],Citation[42] Prompt discontinuation of RAAS blockade led to significant and sustained improvement in four patients, whereas a 50% dose reduction in the fifth patient of candesartan, an ARB, produced similar improvements in eGFR. Mean serum creatinine decreased from 3.4 ± 1.1 (1.8–4.6) mg/dL to 2.1 ± 0.6 (1.4–3.0) mg/dL (p = 0.049, n = 5) 29.6 (20–43) months after RAAS blockade was discontinued in four patients and halved in the fifth. Correspondingly, mean eGFR had increased from 17.0 ± 7.4 (12–30) ml/min/1.73 m2 BSA at enrollment into the study to 24.6 ± 9.5 (17–41) ml/min/1.73 m2 BSA (p = 0.008), 29.6 (20–43) months after withdrawal of RAAS blockade. This syndrome could be pathophysiologically explained on the basis of microvascular renal arteriolar narrowing, mimicking large vessel renal artery stenosis, although it is not demonstrable on MRA or conventional angiography and only evident on renal biopsy.Citation[43] The concept of microvascular renal arteriolar narrowing was first suggested by Raine in 1990, as the presence of microvascular narrowing evident in histological specimens.Citation[43] We submit that microvascular renal arteriolar narrowing, or what we have termed microvascular renal artery stenosis (mRAS), within renal capillaries and arterioles is capable of stimulating a state of enhanced angiotensin II production from the renal juxtaglomerular apparatus in the same way as large renal artery renal artery stenosis lesions do.Citation[43],Citation[44] Notably, these microvascular renal arteriolar narrowing would tend to be more prevalent in the elderly patientsCitation[43]; indeed, three of five patients in our cohort were aged 75 years or older.
As noted earlier, there is a strong evidence-based consensus for renoprotection by RAAS blocking strategies in both diabetic and nondiabetic hypertensives, with and without proteinuria, beyond blood pressure loweringCitation[1–15] (see ). We have observed that in the large randomized placebo-controlled RAAS blockade trials, the patients were relatively younger, in their 50s and 60s, with near normal baseline serum creatinine (1–1.3 mg/dL), fairly preserved GFR, and on less than 25–50% of maximum recommended drug doses (see ). Additionally, some trials were for periods as short as 3–24 months. Remarkably, Suissa et al. described an increased risk ratio of ESRD in 6102 diabetic patients, mean age 66 years, on ACE inhibition versus other antihypertensives, after three years or longer of ACE inhibition.Citation[23] Furthermore, in the CHARM trials, surveillance blood safety analyses available in 2743 North American patients revealed that even though serum creatinine changed slightly between baseline and 6 weeks, serum creatinine had doubled in 82 (6%) of 1263 candesartan patients and 47 (4%) of 1279 placebo patients with surveillance laboratory assessments (p = 0.002)Citation[14] From our findings, and cognizant of these previous observations in the literature,Citation[14],Citation[23] we submit that in certain susceptible older hypertensive diabetic CKD patients, renal failure including ESRD could result from RAAS blockade, despite normal renal arteries and without precipitating factors, even years after starting RAAS blockade. Microvascular RAS is presumed to explain this potentially reversible syndrome.
Table 2 A critical appraisal of the large randomized RAAS blockade trials.Citation[1–5]
MABP in the five patients was higher following discontinuation of RAAS blockade and the substitution of new antihypertensive agents in three of five patients, but this was not statistically significant. The impact, if any, of higher blood pressures in these patients, or an effect of the different substitute antihypertensive agents cannot be interpreted from such a small number of patients. Future larger studies may help answer such questions.
CONCLUSIONS
We acknowledge that this was a single-center experience and we were able to enroll only five patients over a 50-month period. We enrolled only five patients because we employed very stringent exclusive criteria as noted earlier. We did not carry out any renal biopsy procedures, as this was not IRB approved. Similarly, re-challenge with the culprit RAAS blocking agent(s) in these patients was deemed unethical and was therefore not attempted. Even though MRA was not available in all patients, the remaining 95 of 100 (95%) patients had one or another known precipitating factor for worsening renal failure already present and would not have been enrolled (see ). Our observations clearly call for an expanded investigation and study with possibly the inclusion of renal histology examination. Despite these limitations, and given the very significantly improved and remarkably sustained eGFR in all five patients following the discontinuation of RAAS blockade, we surmise that this syndrome must be given due recognition. One patient remains well, dialysis-independent for two years now, and another patient, referred to us ostensibly to start hemodialysis in November 2002, remains well and active, not needing dialysis for four years and going. The other three patients are doing much better, with improved kidney function. The true incidence of this syndrome and the scope of its contribution to the current CKD/ESRD epidemic, for now, can only remain conjectural. We suggest the use of eGFR to monitor kidney function, as this would enable early acknowledgement and appropriate staging of chronic kidney disease.Citation[40] In addition, the prevalence of the microvascular renal artery stenosis can only be speculative at this time.Citation[41],Citation[43] However, it is likely to be more prevalent in the older patients. Until further studies help resolve these questions, it seems prudent to “start low and go slow” with RAAS blockade in the elder patients with closer monitoring. Furthermore, we propose that eGFR in CKD patients on RAAS blockade must be monitored indefinitely, and not just for the first three months following initiation of RAAS blockade. Indeed, as observed in our study, accelerated renal failure in older patients on RAAS blockade could occur, even after years on the same dose of RAAS blockade. These observations are without prejudice to current guidelines, which recommend that small but non-progressive increases in baseline serum creatinine, up to 30%, following the initiation of an ACEI or an ARB should not warrant drug discontinuation.Citation[1],Citation[45] From our study experience, we recommend that RAAS blockade be up-titrated more slowly, particularly in the older patients with close monitoring of eGFR. Such pre-emptive preventative measures would help reduce iatrogenic renal failure in CKD patients on RAAS blockade and will only further optimize both renal and patient outcomes.
DEDICATIONS AND ACKNOWLEDGMENTS
This work is dedicated to the memory of a pleasant, unnamed 74-year-old white female patient of mine with ESRD, who died suddenly at home, watching television, probably from a malignant arrhythmia. Unfortunately, an autopsy was not performed.
We acknowledge the resourcefulness and expertise of Vinay Nijhawan, MD, Interventional Radiologist, Midelfort Clinic, Eau Claire, Wisconsin, in MRA diagnostics.
Notes
*No external funding was involved in this study, and IRB approval was obtained.
REFERENCES
- Onuigbo M, Weir MR. Evidence-based treatment of hypertension in patients with diabetes mellitus. Diabetes Obes Metab. 2003; 5(1)13–26
- Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. The SOLVD Investigators. N Engl J Med. 1992; 327(10)685–691, Erratum: N Engl J Med. 1992; 327(24):1768.
- Lewis EJ, Hunsicker LG, Bain RP, et al. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993; 329(20)1456–1462, Erratum: N Engl J Med. 1993; 330(2):152.
- Ruggenenti P, Perna A, Gherardi G, et al. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet. 1999; 354(9176)359–364
- Packer M, Poole-Wilson PA, et al. Comparative effects of low and high doses of the angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. ATLAS Study Group. Circulation. 1999; 100(23)2312–2318
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999; 341: 709–717
- Mogensen CE, Neldam S, Tikkanen I, et al. Randomized controlled trial of dual blockade of renin-angiotensin system in patients with hypertension, microalbuminuria, and non-insulin dependent diabetes: The Candesartan And Lisinopril Microalbuminuria (CALM) study. BMJ 2000; 321(7274)1440–1444
- Pitt B, Poole-Wilson PA, Segal R, et al. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: Randomized trial—the Losartan Heart Failure Survival Study ELITE II. Lancet. 2000; 355(9215)1582–1587
- Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: Results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet. 2000; 355(9200)253–259, Erratum: Lancet. 2000; 356(9232):860.
- Parving HH, Lehnert H, Brochner-Mortensen J, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001; 345: 870–878
- Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001; 345: 861–869
- Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001; 345: 851–860
- Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003; 349(20)1893–1906
- Pfeffer MA, Swedberg K, Granger CB, et al. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: The CHARM-overall programme. Lancet. 2003; 362(9386)759–766
- Pitt B, Remme W, Zannad F, , for the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003; 348: 1309–1321
- Manolio TA, Cutler JA, Furberg C, et al. Trends in pharmacologic management of hypertension in the United States. Arch Intern Med. 1995; 155(8)829–837
- Carter BL, Malone DC, Ellis SL, et al. Antihypertensive drug utilization in hypertensive veterans with complex medication profiles. J Clin Hypertens (Greenwich). 2000; 2(3)172–180
- Nelson CR, Knapp DA. Trends in antihypertensive drug therapy of ambulatory patients by U.S. office-based physicians. Hypertension. 2000; 36(4)600–603
- Scarsi KK, Bjornson DC. The use of ACE inhibitors as renoprotective agents in Medicaid patients with diabetes. Ann Pharmacother. 2000; 34(9)1002–1006
- Hsu CY, Vittinghoff E, Lin F, et al. The incidence of end-stage renal disease is increasing faster than the prevalence of chronic renal insufficiency. Ann Intern Med. 2004; 141(2)95–101
- Szczech LA, Lazar IL. Projecting the United States ESRD population: Issues regarding treatment of patients with ESRD. Kidney Int Suppl. 2004; 90: S3–S7
- Jones CA, Krolewski AS, Rogus J, et al. Epidemic of end-stage renal disease in people with diabetes in the United States population: Do we know the cause?. Kidney Int. 2005; 67(5)1684–1691
- Suissa S, Hutchinson T, Brophy JM, et al. ACE-inhibitor use and the long-term risk of renal failure in diabetes. Kidney Int. 2006; 69: 913–919
- Silas JH, Klenka Z, Solomon SA. Captopril induced reversible renal failure: A marker of renal artery stenosis affecting a solitary kidney. Br Med J (Clin Res Ed). 1983; 286(6379)1702–1703
- Murphy BF, Whitworth JA, Kincaid-Smith P. Renal insufficiency with combinations of angiotensin converting enzyme inhibitors and diuretics. Br Med J (Clin Res Ed). 1984; 288(6420)844–845
- Jackson B, McGrath BP, Matthews PG, et al. Differential renal function during angiotensin converting enzyme inhibition in renovascular hypertension. Hypertension. 1986; 8: 650–654
- Kalra PA, Mamtora H, Holmes AM, et al. Renovascular disease and renal complications of angiotensin-converting enzyme inhibitor therapy. Q J Med. 1990; 7(282)1013–1018
- Devoy MAB, Tomson CR, Edmunds ME, et al. Deterioration in renal function associated with angiotensin converting enzyme inhibitor therapy is not always reversible. J Intern Med. 1992; 232(6)493–498
- Thomas MC. Diuretics, ACE inhibitors and NSAIDs—the triple whammy. Med J Aust. 2000; 172(4)184–185
- Boyd IW, Mathew TH, Thomas MC. Cox-2 inhibitors and renal failure: The triple whammy revisited. Med J Aust. 2000; 173: 274
- Descombes E, Fellay G. End-stage renal failure after irbesartan prescription in a diabetic patient with previously stable chronic renal insufficiency. Ren Fail. 2000; 22(6)815–821
- Vachharajani TJ, Dacie JE, Yaqoob MM, et al. Detection of occult renovascular disease in unexplained chronic kidney disease. Int Urol Nephrol. 2005; 37(4)793–796
- Schalekamp MADH, Wenting GJ. Angiotensin converting enzyme inhibition in renovascular hypertension: Failure of the stenotic kidney. Lancet. 1984; 1(8374)464
- Hricik DE, Browning PJ, Kopelman R, et al. Captopril-induced functional renal insufficiency in patients with bilateral renal-artery stenosis or renal-artery stenosis in a solitary kidney. N Eng J Med. 1983; 308(7)373–376
- Wenting GJ, Tan-Tjiong HL, Derkx FH, et al. Split renal function after captopril in unilateral renal artery stenosis. Br Med J (Clin Res Ed). 1984; 288(6421)886–890
- Jackson B, Matthews PG, McGrath BP, et al. Angiotensin converting enzyme inhibition in renovascular hypertension: Frequency of reversible renal failure. Lancet. 1984; 1(8370)225–226
- Hollenberg NK. Angiotenisn-converting enzyme inhibition: Renal aspects. J Cardiovasc Pharmacol. 1985; 7: S40–S44
- Hansen HP, Rossing P, Tarnow L, et al. Increased glomerular filtration rate after withdrawal of long-term antihypertensive treatment in diabetic nephropathy. Kidney Int. 1995; 47(6)1726–1731
- Apperloo AJ, de Zeeuw D, de Jong PE. A short-term antihypertensive treatment-induced fall in glomerular filtration rate predicts long-term stability of renal function. Ann Intern Med. 1997; 51(3)793–797
- Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999; 130(6)461–470
- Onuigbo MAC, Onuigbo NT. Late onset renal failure from angiotensin blockade (LORFFAB): A prospective thirty-month Mayo Health system Clinic experience. Med Sci Monit. 2005; 11(10)CR462–CR469
- Onuigbo MAC, Onuigbo NTC. Late-onset renal failure from RAAS blockade. Kidney Int. 2006; 70: 1378–1379
- Raine AE. Angiotensin-converting enzyme inhibition and renovascular disease. Q J Med. 1990; 77(282)997–999
- Ballermann BJ, Onuigbo MAC. Angiotensins. Handbook of Physiology. Section 7, The Endocrine System, Vol III. Endocrine Regulation of water and Electrolyte Balance., JCS Frsy. Oxford University Press, New York 2000; 104–155
- Bakris GL, Weir MR. Angiotensin-converting enzyme inhibitor-associated elevations in serum creatinine: Is this a cause for concern?. Arch Intern Med. 2000; 160(5)685–693