Abstract
It is recognized that cytomegalovirus (CMV) infection in transplant recipients may lead to graft loss. Prophylaxis with acyclovir has therefore gained widespread acceptance, but the debate on whether this intervention improves long term graft survival continues. All patients who received renal grafts at the National Renal Transplant Centre, Dublin, between January 1992 and December 1999 were retrospectively analyzed. During this time period, patients who were CMV positive and/or had received grafts from CMV-positive donors were administered prophylactic oral acyclovir 800 mg thrice daily, adjusted for calculated creatinine clearance, from the first day post-transplantation. This treatment was continued for three months unless the graft failed or the patient developed CMV disease or died. Graft and patient outcomes were compared in recipients who received acyclovir with those who did not. Over the study period, 935 patients received renal transplants in our center, of whom 487 were administered acyclovir. The incidence of CMV disease was 3.3 cases per 100 patients per annum in those who required prophylaxis. Despite prophylaxis, graft outcomes were found to be significantly worse (p value < 0.001) in the group that qualified for acyclovir. We conclude that acyclovir provides incomplete protection from the negative impact of CMV on graft survival.
INTRODUCTION
More effective immunosuppression has translated into better outcomes in renal transplantation.Citation[1] However, such manipulation of the immune system can be associated with potentially fatal infections, including those caused by cytomegalovirus (CMV).Citation[2] The serious nature of diseases caused by CMV, including pneumonia, enteritis, hepatitis, and chorioretinitis, have been the impetus to continued improvement in diagnostic testsCitation[3] and more effective therapeutic agents.Citation[4] In addition, there have been reports of prophylactic oral acyclovir to prevent CMV disease in transplant recipients, with promising results.Citation[5],Citation[6] However, it continues to be matter of debate whether this reduction in CMV disease by acyclovir leads to improved graft survival.Citation[7],Citation[8] Therefore, we retrospectively examined whether acyclovir neutralized the potential effect of CMV on renal transplant outcomes by comparing graft survival in patients who had received the prophylactic regimen with the outcome of CMV negative grafts in CMV negative recipients (D-R-).
PATIENTS AND METHODS
The data of all the patients (n = 935) who received renal allografts at the Beaumont Hospital, Dublin, between January 1992 and December 1999 were retrospectively analyzed. The CMV serologic status of recipients and donors was determined prior to transplantation using enzyme immunoassay, complement fixation, and latex agglutination techniques. CMV disease was diagnosed if there was clinical evidence of pneumonia, hepatitis, enteritis, colitis, chorioretinitis, encephalitis, or fever with leucopenia and/or thrombocytopenia along with positivity of CMV, demonstrable by one or more of the following tests: nPCR, pp65 antigenemia, shell vial assay, CMV culture, and/or new onset of CMV antibodies. CMV infection was defined as serological evidence of CMV exposure with no clinical signs or symptoms of CMV disease.Citation[6],Citation[9] During the study period, it was the policy of our unit to administer oral acyclovir to all recipients who were CMV positive or who received allografts from CMV-positive donors. These patients will henceforth be referred to as the treatment group, and the CMV-negative recipients of CMV-negative organs as the D-R- group.
Acyclovir, 800 mg thrice daily,Citation[5],Citation[10] was administered for three months, commencing on the day of transplantation, unless the patient developed CMV disease, lost the graft, or died. The dosage was adjusted to 800 mg twice daily if the estimated creatinine clearance (Cockroft Gault) was less than 10 mL/min. Patients who remained dialysis-dependent were given one 800 mg dose after each dialysis. Any patient who developed CMV disease was administered i.v. ganciclovir 5mg/kg/day for a minimum of ten days, adjusted to calculated creatinine clearance. In these patients, acyclovir was then administered for a further three months. Recipients were monitored until April 2000 or graft loss, whichever was earlier. The median follow-up period was 3 years and 1 month (50% of recipients having been reviewed for 1 year, 3 months to 5 years, 2 months.)
All recipients were treated with triple immunosuppression consisting of i.v. methyl prednisolone 500 mg daily for three days followed by 20 mg/day of prednisolone daily, azathioprine 1–2 mg/kg/day, or mycophenolate mofetil 500–1000 mg twice daily adjusted to white blood cell and platelet counts, and cyclosporine adjusted to a 12-hour trough level of 200–250 μg/L or tacrolimus adjusted to a 12-hour trough levels of 8–12 ng/L. All highly sensitized patients (defined as those with panel reactive antibodies [PRA] more than 80%) were given anti-lymphocyte antibodies at induction if tolerant of the test dose. Acute rejection and drug toxicity were the only reasons for deviation from this protocol.
The HLA mismatch; the recipients' age, sex, CMV status, and PRA status; the presence of diabetes mellitus; the number of transplants received; episodes of CMV disease; delayed graft function; number of biopsy proven acute rejections; duration of i.v. ganciclovir therapy; and the donor's age, sex, and CMV status were recorded. Graft outcomes in the treatment and the D-R- groups were compared.
Cox proportional hazards modelling were used to calculate the risk of graft loss associated with predictive variables. Kaplan-Meier survival functions were estimated, and graphs were constructed to demonstrate the effect of CMV infection on graft survival. Comparison of clinical and demographic data between the CMV groups was performed using the Wilcoxon rank sum tests for numeric variables and tabulated odds ratios for categorical variables. A significance level of 5% was used to determine the difference between groups.
RESULTS
During the eight-year period from 1992 to 1999, 935 patients received 989 renal transplants, of which 448 recipients were D-R-. The remaining 487 recipients (52.1%) were seropositive for CMV prior to transplantation or received a renal allograft from a CMV-positive donor. All of these individuals received acyclovir prophylaxis. The prevalence of CMV seropositivity was 34.9% among donors and 29.1% among recipients. Heart-beating cadaveric donor allografts were used in 984 instances and live-related in five. Our center did not have a non-heart-beating donor program.
Variables affecting graft survival in this period were studied, and the univariate analysis is presented in . CMV positivity, the number of acute rejections, delayed graft function, recipient age, donor age, and male sex were found to be significant risk factors for graft loss. Multivariate analysis then confirmed the risk engendered by CMV infection (see ). Graft outcomes were found to be significantly worse in the treatment group when compared to those who were D-R- (see ) and remained so after censoring for patient death (p = 0.023, 95% confidence intervals 1.059–2.143).
Table 1 Univariate analysis of factors affecting graft survival
Table 2 Multivariable model of risk factors associated with graft loss
Figure 1. The effect of CMV infection on graft survival, despite acyclovir prophylaxis, adjusted for age and transplant number. X axis: Graft survival in years Y axis: Percentage of grafts surviving.
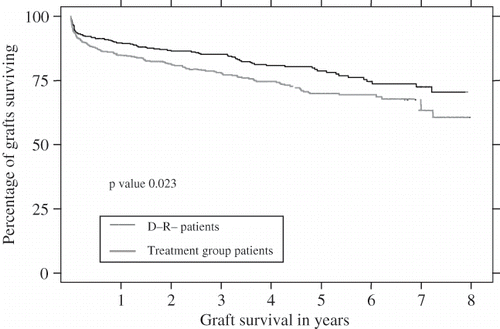
The demographics and characteristics of CMV negative and of CMV positive patients are summarized in . The prevalence of diabetes mellitus was 7.21% among the treatment group recipients and 6.84% in D-R- individuals, but was not significantly different between the two groups (p = 0.819). Similarly, glomerulonephritis as the cause of end stage renal failure did not vary significantly (p = 0.360) between the treatment (30.6%) and D-R- (33.33%) patients. Mortality was significantly higher (p = 0.022) in the patients who qualified for acyclovir prophylaxis (12.4% versus 7.9%), as was the number of recipients who died with a functioning graft (14.18% versus 8.68%, p = 0.014), although no specific risk factor other than CMV infection could be identified.
Table 3 Comparison of treatment group versus D-R- patients
The overall incidence of acute rejection was 26.10% but did not vary significantly between the treatment (24.95%) and D-R- (27.35%) groups. The number of acute rejection episodes was noted to be a significant risk factor for graft loss but was not associated with CMV positive status (p = 0.213). Rejection was the cause of graft loss in 7.81% of the D-R- group and in 10.88% of the treatment group (p = 0.102).
The incidence of CMV disease was higher in the treatment group—3.3 versus 1.3 cases per 100 patients per annum—but did not affect graft survival significantly (p = 0.394), nor did the duration of i.v. ganciclovir therapy (p = 1.01).
Subgroup analysis of CMV status revealed similar outcomes for grafts from CMV-positive donors to CMV-positive recipients and from CMV-positive donors to CMV-negative recipients (see and and ). The negative impact of CMV positivity was least in CMV-positive recipients who received grafts from CMV-negative donors (D-R+). This effect was so pronounced in our series that there was no significant difference in graft half-life in this group when compared to the D-R- recipients.
Figure 2. The effect of donor and/or recipient CMV infection on graft survival. X axis: Graft survival in years Y axis: Percentage of grafts surviving.
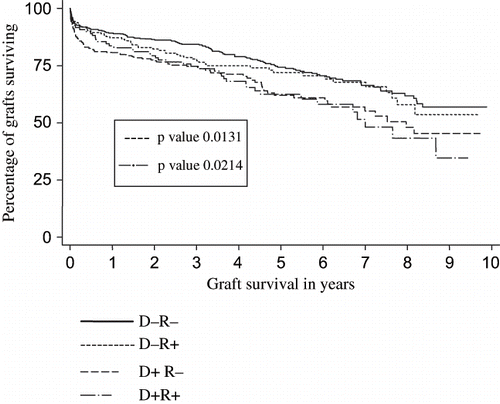
Table 4 Comparison of different subgroups of CMV-positive or -negative transplants
Table 5 Graft survival over one, three, and five years
DISCUSSION
The human herpes viruses account for a significant proportion of the infections seen in renal transplant recipients,Citation[2] only some of which are due to the reactivation of latent infections following immunosuppression.Citation[11] Human herpes virus 5, otherwise known as CMV, is a common pathogen in this settingCitation[2] and adversely impacts graft survival.Citation[7] Effective prophylactic regimens with acyclovir or ganciclovir have reduced the incidence of CMV disease,Citation[5],Citation[6],Citation[2] but it is unclear whether they have also neutralized the negative impact of CMV infection on renal transplant half-life. Better outcomes with prophylaxis were noted by Bock et al.,Citation[13] who observed that any one of the three modalities studied—acyclovir, ganciclovir, or immunoglobulin prophylaxis—improved three-year graft survival in pediatric recipients. Trials in adults have also demonstrated that prophylacticCitation[14],Citation[15] or pre-emptive ganciclovirCitation[16] treatment reduced the risk of earlier graft failure. However, the growing number of reports describing ganciclovir resistance in CMV isolatesCitation[17],Citation[18] suggests that reserving this drug for treatment of CMV disease may be a wiser long-term option than using it for prophylaxis. Other antiviral agents will therefore be required for prevention.
In our eight-year study, we noted a low prevalence rate of CMV seropositivity among both donors and recipients. This observation approximates the 30% prevalence noted in blood donors by the Department of Health, Ireland.Citation[19]
We also found that acyclovir prophylaxis did not result in outcomes comparable to those in uninfected recipients, except in CMV-positive individuals who received CMV-negative allografts. Both recipient and graft survival were worse in individuals who qualified for acyclovir prophylaxis, despite recipient and donor characteristics that were similar to the CMV-negative group. The trend of poorer graft outcomes in the treated patients remained significant after censoring for individuals who died. Our findings indicate that acyclovir prophylaxis is not sufficiently effective, or that the duration of treatment is too short to neutralize the adverse impact of CMV infection on graft survival. Our observations lend support to the results of the study conducted by Lo et al., who noted that D-R- recipients of simultaneous pancreas kidney transplants had better patient and graft survival rates than CMV-positive patients who received ganciclovir prophylaxis.Citation[20] In order to identify the reasons for our findings, we evaluated three factors that could mediate the effect of CMV infection on kidney transplants:
An increased incidence of acute rejection episodes is thought to be one mechanism by which CMV affects transplant half-life.Citation[21],Citation[22] This phenomenon is probably caused, at least in part, by the virus's ability to up-regulate HLA expression within the graft and thereby increase the immunogenicity of the transplant.Citation[2],Citation[11] Our study, however, showed no significant association between CMV infection and acute rejections, which is similar to the observations made by Dickenmann et al.Citation[23] It is possible that the routine use of more effective immunosuppression has reduced the incidence of acute rejections,Citation[1] without halting the process of chronic, low-grade immune-mediated damage. In addition, the cumulative effects of a chronic rejection are likely to be better demonstrated by a long follow-up period, as in our study. The poorer graft half-life in patients who were transplanted with CMV-positive organs when compared to those who received uninfected allografts suggests that the fresh antigen challenge presented by CMV-infected transplants plays a more important role in determining graft survival than the recipients' previous exposure to CMV.
CMV nephropathy can cause graft dysfunction,Citation[24] but we found no significant association between the incidence of CMV disease and graft failure. Interestingly, despite improved prevention of disease with ganciclovir, Rubin et al. showed no significant difference in acute rejection episodes when compared to recipients prophylaxed with acyclovir.Citation[10] These findings and the demonstration of persistent CMV infection despite prophylaxisCitation[25] suggest that CMV disease as currently defined is not the cause of lower graft survival, whereas subclinical CMV infection may play a significant role in graft loss.
Lastly, the nephrotoxic potential of ganciclovir is unlikely to be a significant factor affecting transplant survival as, in our study, increased duration of therapy was not associated with greater graft loss.
The growing body of literature suggesting increased efficacy prompted a change in our CMV prophylaxis to valacyclovirCitation[22] in January 2000.
To conclude, D-R+ patients received acyclovir prophylaxis and demonstrated a graft survival similar to that of D-R- recipients. Acyclovir prophylaxis does not neutralize the negative impact of CMV on graft survival in patients who receive CMV-positive organs, regardless of their CMV status prior to transplantation. Our search for effective alternatives continues.
Notes
*Presented in part at the ASN/ISN World Congress of Nephrology, San Francisco, California, USA, 2001.
REFERENCES
- Hariharan S, Johnson CP, Bresnehan BA, Taranto SE, McIntosh MJ, Stablein D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000; 342: 605–612
- Smith SR, Butterly DW, Alexander BD, Greenberg A. Viral infections after renal transplantation. Am J Kidney Dis. 2001; 37: 659–676
- Tanabe K, Tokumoto T, Ishikawa N, et al. Comparative study of cytomegalovirus (CMV) antigenemia assay, polymerase chain reaction, serology, and shell vial assay in the early diagnosis and monitoring of CMV infection after renal transplantation. Transplantation. 1997; 64: 1721–1725
- Brennan DC. Cytomegalovirus in renal transplantation. J Am Soc Nephrol. 2001; 12: 848–855
- Balfour HH, Jr, Chace BA, Stapleton JT, Simmons RL, Fryd D S. A randomized, placebo-controlled trial of oral acyclovir for the prevention of cytomegalovirus disease in recipients of renal allografts. N Engl J Med. 1989; 320: 1381–1387
- Barkholt L, Lewensohn-Fuchs I, Ericzon BG, Tyden G, Andersson J. High-dose acyclovir prophylaxis reduces cytomegalovirus disease in liver transplant patients. Transpl Infect Dis. 1999; 1: 89–97
- Helantara I, Koskinonen P, Finne P, et al. Persistent cytomegalovirus infection in kidney allografts is associated with inferior graft function and survival. Transpl Int. 2006; 19: 893–900
- McLaughlin K, Sandhu S, Wu C, Muirhead N, Hollomby D, Jevnikar A. Transplanting kidneys from CMV-seropositive donors to CMV-seronegative recipients is not associated with poorer renal allograft function or survival. Nephrol Dial Transplant. 2005; 20: 176–180
- Conlon PJ, Jr, Carmody M, Donohoe J, Spencer S, Smyth E, Walshe JJ. Cytomegalovirus infection as a complication of OKT3 therapy in kidney transplant recipients. Ir J Med Sc 1992; 161: 630–632
- Rubin RH, Kemmerly SA, Conti D, et al. Prevention of primary cytomegalovirus disease in organ transplant recipients with oral ganciclovir or oral acyclovir prophylaxis. Transpl Infect Dis. 2000; 2: 112–117
- Snydman DR, Rubin RH, Werner BG. New developments in cytomegalovirus prevention and management. Am J Kidney Dis. 1993; 21: 217–228
- Becker BN, Becker YT, Leverson GE, Simmons WD, Sollinger HW, Pirsch JD. Reassessing the impact of cytomegalovirus infection in kidney and kidney-pancreas transplantation. Am J Kidney Dis. 2002; 39: 1088–1095
- Bock GH, Sullivan EK, Miller D, et al. Cytomegalovirus infections following renal transplantation-effects on antiviral prophylaxis: A report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr Nephrol. 1997; 11: 665–671
- Opelz G, Dohler B, Rubenstroth A. Cytomegalovirus prophylaxis and graft outcome in solid organ transplantation: A collaborative transplant study report. Am J Transplant. 2004; 4: 928–936
- Ricart MJ, Malaise J, Moreno A, Crespo M, Fernandez-Cruz L. Cytomegalovirus: Occurrence, severity, and effect on graft survival in simultaneous pancreas-kidney transplantation. Nephrol Dial Transplant. 2005; 20(Suppl. 2)25–32
- Schneeberger H, Aydemir S, Muller R, et al. Hyperimmunoglobulin prophylaxis, monitoring and preemptive ganciclovir treatment eliminate the risk of CMV infection to improve patient and renal allograft survival. Transpl Int. 2000; 13(Suppl. 1)S354–S358
- Limaye AP, Corey L, Koelle DM, Davis CL, Boeckh M. Emergence of ganciclovir-resistant cytomegalovirus disease among recipients of solid-organ transplants. Lancet. 2000; 356: 645–649
- Ketteler M, Preuschof L, Mertz A, et al. Fatal cytomegalovirus pneumonia after preemptive antiviral therapy in a renal transplant recipient. Clin Nephrol. 2000; 54: 418–424
- Bonnar J, Browne P, Cahill M, et al. A guideline for transfusion of red blood cells in surgical patients. National Blood Users Group, Department of Health, DublinIreland 2000; 1–20
- Lo A, Stratta RJ, Egidi MF, et al. Patterns of cytomegalovirus infection in simultaneous kidney-pancreas transplant recipients receiving tacrolimus, mycophenolate mofetil, and prednisone with ganciclovir prophylaxis. Transpl Infect Dis. 2001; 3: 8–15
- Toupance O, Bouedjoro-Camus MC, Carquin J, et al. Cytomegalovirus-related disease and risk of acute rejection in renal transplant recipients: A cohort study with case-control analyses. Transpl Int. 2000; 13: 413–419
- Reisching T, Jindra P, Mares J, et al. Valacyclovir for cytomegalovirus prophylaxis reduces the risk of acute renal allograft rejection. Transplantation. 2005; 15: 317–324
- Dickenmann MJ, Cathomas G, Steiger J, Mihatsch MJ, Thiel G, Tamm M. Cytomegalovirus infection and graft rejection in renal transplantation. Transplantation. 2001; 71: 764–767
- Boratynska M, Banasik M, Watorek E, Patrzalek D, Szyber P, Klinger M. Influence of cytomegalovirus disease on early and late renal graft function. Transplant Proc. 2006; 38: 147–150
- Isenberg AL, Shen GK, Singh TP, Hahn A, Conti DJ. Failure of ganciclovir prophylaxis to completely eradicate CMV disease in renal transplant recipients treated with intense antiHrejection immunotherapy. Clin Transplant. 2000; 14: 193–198