Abstract
The Optimal Renal Anaemia Management Assessment trial prospectively examined the impact of implementing European Best Practice Guidelines on outcomes in the management of renal anemia. Baseline data give an insight to standards of clinical care and provide a basis for a future comparison of guideline target attainment with final results. Fifty-three centers from eight European countries enrolled 739 patients with stage II–V chronic kidney disease who were either anemic (hemoglobin <11 g/dL) or treated with erythropoiesis-stimulating agents and/or iron supplementation. Patients were followed over 6–8 months in centers that were randomly allocated to either group A or B (i.e., with or without a computerized clinical decision support tool after baseline). The latter provided guideline-based recommendations to physicians based on patient anemia-related data. We report patient characteristics and hemoglobin values from baseline and the prestudy period, focusing on regional differences. In all, 81% of patients were dialysis-dependent. Baseline mean hemoglobin values in dialysis patients were significantly higher in Western (11.8 g/dL) vs. Eastern Europe (10.6 g/dL; p < 0.0001). Similar proportions of patients (∼50%) had mean hemoglobin 10–12g/dL suggesting Eastern European patients are treated to lower Hb levels. The guideline ferritin target was achieved by 85% of dialysis and 52% of non-dialysis patients; 81% of dialysis and 78% of non-dialysis patients attained the transferrin saturation target. Most patients (96%) were receiving erythropoiesis-stimulating agents. Anemia management in patients with chronic kidney disease shows considerable regional differences across Europe, and target attainment remains suboptimal in many European nephrology centers after the revised 2004 guidelines.
INTRODUCTION
Renal anemia is common in patients with chronic kidney disease (CKD), and its negative impact on quality of life, morbidity and mortality is well established.Citation[1] Despite a lack of detailed data regarding the relative importance of the different factors that contribute to the development of anemia in the early stages of CKD, erythropoietin deficiency appears to be the most important reason for the subsequent decline in hemoglobin (Hb) levels.Citation[1],Citation[2]
The use of erythropoiesis stimulating agents (ESAs) for the treatment of renal anemia is now standard, and in 2006, iron and ESA treatment accounted for 10 billion USD of healthcare spending worldwide.Citation[3],Citation[4] The beneficial effects of this class of agents in patients with CKD have been well documented and include a decreased demand for transfusions,Citation[5] improved physical performance,Citation[6] and better quality of life.Citation[5],Citation[7] Evidence also suggests that anemia correction to approximately 11–12 g/dL leads to the partial regression of left ventricular hypertrophy and reduces mortality rates.Citation[8] There has been much discussion as to the optimum Hb treatment target with reports that no additional quality of life benefits are achieved with higher Hb targets (i.e., ≥13.5 g/dL) and that high target Hb (i.e.,12–16 g/dL) is associated with increased all-cause mortality.Citation[4],Citation[9]
Targets for the optimal treatment of renal anemia have been set out in Europe (1999, updated 2004),Citation[2],Citation[10] the United States (2002, revised 2006),Citation[8],Citation[11] and Australia (revised 2005).Citation[12] Despite some improvements in the management of renal anemiaCitation[13] following the publication of these guidelines, studies assessing their impact show substantial variations in clinical practice and patient outcomes between centers and between countries.Citation[5],Citation[14]
In 2003, a cross-sectional survey of anemia management of 8,100 dialysis patients in eight European countries made a comparison with a similar assessment carried out in 1998, prior to the publication of the first European Best Practice Guidelines (EBPG).Citation[15] The reported increase in the percentage of patients achieving the target Hb was attributed to a concurrent increase in ESA dose. However, the percentage of patients with Hb level >11 g/dL showed considerable inter-country variation, ranging from 79% in Switzerland to 37% in Poland. A similar degree of international variation was reported in a large (N = 11,041), multinational, prospective observational study conducted in two phases, the first between 1996–2001 (DOPPS I) and the second between 2002–2003 (DOPPS II).Citation[5]
Anemia management in non-dialysis patients with CKD stages II–IV is suboptimal compared with the EBPG targets, and marked inter-country variations exist.Citation[16]
The objective of the Optimal Renal Anaemia Management Assessment (ORAMA) study was to prospectively assess the impact of implementing 2004 revised EBPG on outcomes in the management of renal anemia. Computerized clinical decision support (CDS) systems, which are used increasingly in clinical practice, were provided at certain ORAMA treatment centers.Citation[17] These tools “compare patient characteristics with a knowledge base and then guide a healthcare provider by offering patient and situation-specific advice,”Citation[18],Citation[19] and have been shown to be beneficial in improving drug dosing, enhancing preventive care, and managing specific disease states.Citation[17] CDS systems provide the opportunity to examine the impact of implementing best practice guidelines on clinical outcomes, and ORAMA final results will give an indication of how readily such tools are incorporated into everyday clinical practice.
Currently, very few reports of trends in anemia management between Eastern and Western Europe are available. The cultural, political, and economical differences between these two regions may influence clinical practice and thus patient outcomes; ORAMA final results will reveal whether guideline implementation via CDS systems can be used to minimise these differences. In this article, prestudy Hb values and baseline data are reported and analyzed descriptively with a primary focus on regional differences between Eastern and Western Europe. Comparisons are made to targets set out by the revised EBPG on anemia management in CKD.
METHODS
Study Design
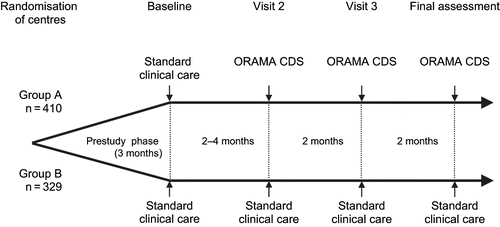
ORAMA was a randomized, prospective study involving nephrology centers from Western and Eastern European countries. Prior to enrolment, participating centers from Bulgaria, Croatia, Germany, Italy, Latvia, Poland, Romania, and Serbia & Montenegro were randomly allocated to and matched between the study groups. Centers in Group A had access to a computerized clinical decision support (CDS) system based on the EBPG while centers in Group B did not. At baseline, none of the centers had access to the CDS. The ORAMA CDS tool generated EBPG-based prompts based on input of individual patient data. Physicians were not obliged to follow CDS recommendations, and ultimately treatment decisions were made according to the physician's judgment.
Eligible patients were assessed at baseline with respect to demographics, concomitant diseases, renal function, dialysis status, and blood pressure. In addition to baseline measurements, Hb values were recorded on up to three occasions over a period of three months prior to enrolment (prestudy phase; see ). Attainment of EBPG targets for Hb (>11 g/dL), serum ferritin (>100 μg/L), and hypochromic red cells (<10%), or transferrin saturation (TSAT) (>20%) were primary endpoints. The proportion of patients attaining at least one primary endpoint, investigator adherence with the CDS recommendations, use of iron supplementation (dose, route, administration), and use of ESAs (dose, route, administration) will also be analyzed.
Study Population
ORAMA enrolled adult patients with stage II–V CKD. Enrolment was complete in September 2005. The presence of chronic renal anemia (Hb <11 g/dL) or treatment with ESA and/or iron supplementation was prerequisite to inclusion. Subjects were also eligible to participate following renal transplantation if the above criteria were met.
Important exclusion criteria included signs of acute or chronic infection or inflammation (plasma CRP concentration >15 mg/L), pregnancy, severe hyperparathyroidism, non-renal anemia, hemoglobinopathies, severe co-morbidities, and anticipated poor compliance.
All patients gave informed consent, and the protocol was approved by the responsible ethical review committees at all participating centers. The study was conducted according to the modified Declaration of Helsinki.
Statistical Methods
Assuming normally distributed data and an inter-subject coefficient of variation of 0.15 for Hb concentration, based on previous measurements in CKD patients, the inclusion of 500 subjects gives a >90% power to detect a 0.5 g/dL difference in Hb concentration between groups, and a >98% power to detect a 1.0 g/dL difference.
Baseline data are analyzed descriptively. Regional differences were tested for significance using the χ2 test, the Wilcoxon-Mann-Whitney test, or the t-test.
RESULTS
Demographics and General Medical Status
The patient population included 346 CKD patients in treatment centers in two Western European countries (i.e., Germany and Italy) and 393 patients in 32 centers in six Eastern European countries (i.e., Bulgaria, Croatia, Latvia, Poland, Romania, and Serbia & Montenegro).
Overall, the patient demographics and baseline medical status were as expected for a population of patients with stages II-V CKD (see ). Characteristics were generally similar in both randomization groups, although patients in group A were slightly older (mean age in years: A,61; B,57), and there was some variation in major causes of CKD. There were no important differences in gender split, weight, height, or blood pressure between Western and Eastern European dialysis patients. However, mean age was higher in Western (64 years) than in Eastern Europe (55 years). In Western Europe, significantly more patients (26%) had hypertensive nephrosclerosis as the underlying cause of CKD than in Eastern Europe (14%; p < 0.0001). Diabetic nephropathy was more often a cause of CKD in Western than in Eastern Europe, where glomerulonephritis and pyelonephritis were considerably more frequent causes of CKD than in the West. Glomerulonephritis was the underlying cause in 57% of all CKD cases in Romania, while it accounted for only 34% of cases in the rest of Eastern Europe. This high incidence may reflect the fact that patients taking part in ORAMA in Romania were on average younger than those in other countries (mean age in years: Romania, 52; all countries, 59).
Table 1 Demographics and baseline characteristics of all patients by region
Approximately half (47%) of all patients had concomitant cardiovascular diseases, with a higher proportion in Western (52%) than in Eastern Europe (43%; see ). Diabetes was the second most frequent concomitant medical condition, and was significantly more frequent in Western (40%) than in Eastern Europe (16%; p < 0.0001), with country specific figures ranging from 42% in Germany to 3% in Romania and Serbia & Montenegro.
Significantly more patients were treated with ACE (angiotensin-converting enzyme) inhibitors and beta blockers in Western (51%, 51%) than in Eastern Europe (37%, 34%, respectively; p < 0.0001; see ). In contrast, other antihypertensive medications were prescribed to significantly more patients in the East (50%) than in the West (33%; p < 0.0001).
Eighty-one percent (599) of patients enrolled were undergoing dialysis at baseline. The proportion of dialysis patients was somewhat lower in the West (77%) than in the East (85%; see ). The majority of patients on dialysis in both regions received hemodialysis (West, 91.4%; East, 97.3%), apart from in Italy, where, in this study, the proportion receiving hemodialysis and peritoneal dialysis was approximately equal. Among those patients receiving iron therapy, a higher proportion received it orally in Italy than in most other countries (28% vs. 11%). However, patient numbers were low, and it is unlikely that this distinction in the Italian cohort had a substantial impact on other patient characteristics at baseline and over the course of the study. Hemodialysis was administered three times a week to more than 90% of patients in both regions and was adequate (Kt/V >1.2) in more patients in Western (86%) than in Eastern Europe (67%). Mean Kt/V was significantly higher in Western (1.4), than in Eastern Europe (1.3; p < 0.0001). The mean duration of dialysis prior to study entry was similar in the two regions, around 50 months, with considerable inter-country variation ranging from 20 months in Latvia to 97 months in Romania.
The remaining 140 patients with CKD stages II–IV were recruited in Bulgaria, Germany, and Italy.
Anemia Treatment
Among dialysis patients, all but two received ESA therapy in the West (∼100%), compared with 94% in the East (see ). In Eastern Europe, the percentages were lowest in Serbia & Montenegro (83%) and Romania (93%), while the proportions of patients receiving ESAs in other Eastern European countries were similar to those in Western Europe.
Table 2 Anemia treatment among dialysis patients by region
Seventy-five percent of dialysis patients were treated with epoetin beta (NeoRecormon®), with similar proportions in the two regions. Among dialysis patients treated with epoetin beta, 49% of Western and 89% of Eastern European patients received it subcutaneously (SC; see ). Eight percent of the dialysis patients receiving ESA therapy were being treated with epoetin beta IV in Eastern Europe and received a median weekly dose of 2000 IU, while their Western European counterparts (38% of dialysis patients on ESA therapy) received 6000 IU. Only 17% and 8% of patients received epoetin alfa or darbepoetin alfa, respectively.
Among non-dialysis patients in this study, the use of ESA was extensive, close to 100%. Epoetin beta SC (95%) was the predominant treatment administered with a median weekly dose of 5000 IU. For non-dialysis patients in Western Europe, a considerably lower median weekly epoetin beta SC dose (3000 IU) was reported than for those in Eastern Europe (6000 IU).
Almost two-thirds of dialysis patients received iron therapy at baseline, with similar proportions in Western (66%) and Eastern Europe (64%; see ). Iron was primarily administered IV in both regions, with slightly higher IV iron use in Western Europe. The high proportion of IV iron use in Western Europe was driven by Germany, the largest representative country of that region, where 96% of patients receiving iron products were treated IV compared with 61% of patients in Italy.
Iron use among non-dialysis patients was significantly higher in Eastern Europe (60%), represented by Bulgaria, than in Western Europe (31%; p = 0.0007). Among those receiving iron therapy, IV iron was administered to nearly two-thirds of non-dialysis patients in Eastern Europe and one-fifth of non-dialysis patients in Western Europe.
Hematological Indices
Dialysis Patients
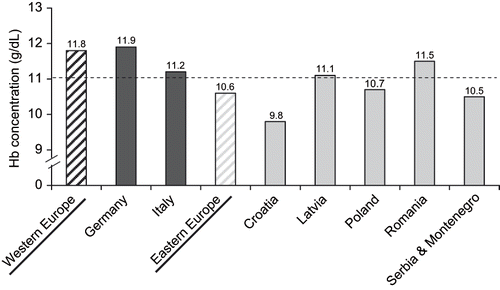
Among dialysis patients, the mean Hb value at baseline was 11.1 g/dL. It was significantly higher in Western than in Eastern Europe (West: 11.8 g/dL; East: 10.6 g/dL; p < 0.0001) with much inter-country variation (see and ). For instance, in Croatia, the baseline mean Hb was particularly low, only 9.8 g/dL, while in Romania, the baseline mean Hb was 11.5 g/dL, well above the regional average of 10.6 g/dL for dialysis patients.
Table 3 Hematological parameters by region for dialysis and non-dialysis patients
The mean of four Hb measurements (three taken over the three-month prestudy phase and one at baseline) was 11.7 g/dL in Western and 10.5 g/dL in Eastern Europe. In Western Europe, the proportion of dialysis patients achieving the recommended EBPG Hb target of >11 g/dL with mean baseline and prestudy measurements was considerably higher (75%) than in Eastern Europe (30%). However, the proportion of dialysis patients with a stable Hb value (in the range of 10–12 g/dL) during the prestudy phase and at baseline was similar in the two regions (West: 52%; East: 55%). Inter-country variations were also reflected in the proportion of patients with mean prestudy and baseline Hb above the EBPG lower Hb target; among dialysis patients, the >11 g/dL Hb target was achieved by 2% of patients in Croatia, 78% in Germany, and 70% in Romania. The proportion of dialysis patients with mean Hb (over the prestudy phase and at baseline) between 10–12 g/dL also varied considerably between countries, the lowest being in Croatia (33%) and the highest in Romania (73%).
Only 36% of dialysis patients had each of the four Hb measurements taken over the prestudy phase and at baseline >11 g/dL (see ). A significant difference was observed between Western and Eastern Europe in meeting this Hb target; among dialysis patients, 57% were above the EBPG target in the West and only 19% in the East (p < 0.0001). Less than 30% of patients in both regions (West: 26%; East: 29%) had each of the four Hb values (recorded over the prestudy phase and at baseline) within the 10–12 g/dL range.
A higher proportion of patients had baseline Hb >13 g/dL in Western than in Eastern Europe (West: 13%; East: 4%).
Non-Dialysis Patients
In non-dialysis patients, Hb at baseline was lower in Western (11.3 g/dL, i.e., 11.5 g/dL in German patients; 10.0 g/dL for Italian patients) than in Eastern Europe (represented by Bulgaria; 11.6 g/dL), and a similar difference could be seen based on mean Hb over the prestudy phase and at baseline (West: 11.2 g/dL; East: 11.4 g/dL; see ). The proportion of non-dialysis patients with baseline Hb >11 g/dL was similar, approximately 60%, in both regions. The proportion of patients with stable Hb (10–12 g/dL) at baseline and at each of the three prestudy measurements was higher in Western than in Eastern Europe (West: 34%; East: 22%).
Iron Status
Mean serum ferritin at baseline was significantly higher among dialysis patients in Western (602 μg/L) than in Eastern Europe (379 μg/L; p < 0.0001; see ), ranging from 262 μg/L in Croatia to 664 μg/L in Germany. In Western Europe, significantly more dialysis patients (91%) had serum ferritin level >100 μg/L (EBPG target value) than in Eastern Europe (80%; p < 0.0005). Serum ferritin measurements were not available for 5% of dialysis patients in Western and 4% of dialysis patients in Eastern Europe.
Mean transferrin saturation (TSAT) at baseline was similar in Western (28%) and Eastern (31%) European countries. TSAT data were not available for 27% of dialysis patients in Western and 16% in Eastern Europe. The proportion of patients with TSAT >20% (EBPG target value) was significantly lower in Western European (74%) than in the Eastern European dialysis patients (86%; p < 0.0014).
DISCUSSION
ORAMA is the first prospective trial to assess the impact of EBPG recommendations on patient outcomes. Prestudy and baseline data reported here highlight important regional differences in standard clinical care across Europe.
Based on retrospective and cross-sectional international trials, the management of anemia in patients with CKD has improved over time and with the evolution of best practice guidelines, although considerable inter-country variations have been documented.Citation[1],Citation[5],Citation[15],Citation[16],Citation[20]
In ORAMA, Western and Eastern European patient demographics and baseline characteristics were generally similar, although some differences were apparent. Patients enrolled were slightly older in Western Europe than in Eastern Europe. Dialysis efficiency based on Kt/V was significantly higher in Western than Eastern Europe (West: 1.4; East: 1.3; p < 0.0001). However, mean Kt/V in both regions exceeded the EBPG target of >1.2, the level required to maximize the effects of ESA therapy. Hypertensive nephrosclerosis and diabetic nephropathy were the main causes of CKD in Western Europe, while in Eastern Europe, glomerulonephritis and pyelonephritis were the most frequent causes of CKD. The most prevalent concomitant diseases in ORAMA were cardiovascular disease and diabetes.
Around four-fifths of patients in ORAMA were on dialysis at baseline, and the majority was receiving hemodialysis rather than peritoneal dialysis.
In ORAMA, a higher proportion of patients received ESA than in the European DOPPS.Citation[1] ESA use was dominated by epoetin beta and was extensive in both regions. In Western Europe, 49% of dialysis patients receiving epoetin beta were treated SC, compared with 89% in Eastern Europe; these figures represent a move toward SC ESA usage compared with earlier studies. In both regions, 97% of patients receiving epoetin alfa were treated IV. These findings correspond with the EBPG for the treatment of anemia in dialysis patients, which recommend that for economic reasons, epoetin beta be used and administered SC, while epoetin alfa is not licensed for SC administration in patients with CKD in most European countries because of the potential risk of pure red cell aplasia (PRCA). Among dialysis patients, the median weekly epoetin beta dose in Western Europe was higher than in Eastern Europe. While higher weekly dose did not correspond directly with higher mean Hb, this may explain the finding that the overall proportion of patients with Hb >11 g/dL was higher in Western than in Eastern Europe. Darbepoetin alfa doses were also higher in the West; however, only 7% of all patients received such therapy.
Sixty-six percent of dialysis patients in Western and 64% of patients in Eastern Europe were receiving iron therapy. These figures are lower than EBPG targets, which recommend that patients with renal anemia and CKD who are on ESA therapy should be given supplementary iron in order to maintain or reach Hb targets.Citation[2] Compared with DOPPS European countries, IV iron use was more frequent,Citation[1] although considerable inter-country variations exist with regard to route of administration in both studies. In contrast to the trend for ESA therapy, generally fewer patients in Western Europe received iron than in the East, with more marked regional difference among non-dialysis patients (see ).
TSAT levels were slightly less favorable in Western Europe compared with the East, although data were unavailable for 27% of patients in the West and for 15% in the East. Serum ferritin was higher in Western than in Eastern European patients, with inter-country variation. A higher proportion of patients in Western than in Eastern Europe had serum ferritin values above the EBPG target (>100 μg/L). As serum ferritin correlates directly with the total amount of iron stored in the body, Western European TSAT values are lower than may be expected in view of the serum ferritin levels in this region. In interpreting these results, it should be considered that iron data were incomplete for many patients.
Dialysis patients in Western Europe had higher mean Hb values than in Eastern Europe at baseline, although there was considerable inter-country variation. Correspondingly, a higher proportion of Western patients met the EBPG Hb target than Eastern European patients. However, the proportion of patients with Hb stable at 10–12 g/dL was similar in the two regions. That 13% of patients in Western Europe had Hb >13g/dL, in spite of the possible adverse effects associated with higher target Hb, suggests a need for treatment guidelines to address the issue of the upper Hb target. It is noteworthy that differences in regional averages are less apparent when the criteria are applied to all four individual Hb measurements (three taken during the three-month prestudy phase and one at baseline). This suggests that although EBPG targets are better met in Western than in Eastern Europe, Hb attainment to the above guideline target is not maintained even in the West. These are the first findings from a prospective study to highlight that mean Hb value can be misleading. Furthermore, early Hb measurements that were abnormal are likely to have been addressed so that subsequent measurements show improved Hb levels and reporting mean Hb would skew the impression of prestudy and baseline Hb status. As a response to ESA therapy can vary over time, a clear picture of Hb status is best obtained by individually analyzing each measurement in a series with respect to EBPG targets.
Mean Hb at baseline and over the prestudy period among dialysis patients was 11.1 g/dL in ORAMA, which is slightly higher than documented in European countries of DOPPS.Citation[1] The proportion of patients with Hb >11 g/dL in ORAMA was comparable with DOPPS Europe. It should be taken into account, however, that in the European DOPPS, only Western European countries were included, and only one country was common to both studies. Hb measurements in the ORAMA study for Western Europe showed improvement compared with DOPPS Europe.
A retrospective cohort study analyzing the management of 4,333 non-dialyzed CKD patients across Europe, Israel, and South Africa between 1999 and 2000 (PRESAM)Citation[16] reported that the majority of these patients had been under specific nephrologist care for more than 12 months prior to the initiation of dialysis, but only 27% received ESAs. Moreover, EBPG targets for Hb concentration were achieved by 22% of non-dialysis patients in Western European countries and only 10% of patients in Eastern European countries.Citation[16] The inter-country variation in the baseline characteristics of ORAMA non-dialysis patients suggests underlying differences in clinical practice. An analysis of final results may reveal possible causes of this variation and to what extent it may be lessened by the EBPG-based CDS tool. Based on previous reports, it is possible that the relatively high use of ESAs in the ORAMA non-dialysis population is more a reflection of the inclusion criteria for the study than of standard clinical care of these patients.Citation[16] It is noteworthy that non-dialysis patient numbers were low in ORAMA and that Eastern Europe was represented only by patients from Bulgaria, and Western Europe only by Germany. Therefore, regional differences seem to be reflective of differences between these two countries.
The considerable inter-country variations in hematological indices may be related to the regional differences in ESA dose, iron use, and also the efficiency difference in dialysis based on Kt/V observed at ORAMA baseline. The regional differences in the use of therapeutic substances, both ESAs and other medication for the treatment of concomitant diseases, may well reflect differences in reimbursement systems.
A limitation of the ORAMA study is that Western Europe is represented by only two countries, although the Western and Eastern patient population is similar (West: 346; East: 393). In comparing ORAMA with previous studies, it should be remembered that only patients who were anemic or receiving ESAs and/or iron therapy were enrolled in ORAMA.
CONCLUSION
ORAMA baseline data indicate some improvements (with regional variation) in anemia management compared with previous studies, but treatment is still suboptimal compared with EBPG targets.
Final results will provide valuable insights into how adherence to guidelines affects the outcome of anemia management in both European regions.
ACKNOWLEDGMENTS
We would like to thank all study investigators and clinical monitors of the study. Without their continuous commitment to the collection of high quality data this study would not have been possible.
Andrzej Wiecek has received travel grants and honoraria and has participated in clinical trials supported by F. Hoffmann-La Roche Ltd, Jannsen-Cilag, and Amgen. Adrian Covic has received honoraria for lectures and advisory boards for F. Hoffmann-La Roche Ltd and for advisory boards for Fresenius Medical Care. Francesco Locatelli has received honoraria for lectures and panels from F. Hoffmann-La Roche Ltd, Amgen, Dompé Biotec, and Shire Pharmaceuticals. Iain C. Macdougall has received research grants/consultancy fees, speaker fees, and honoraria from Ortho Biotech, F. Hoffmann-La Roche Ltd, and Amgen, as well as consultancy fees and honoraria from Shire and Affymax. This study was sponsored by F. Hoffmann-La Roche Ltd.
Notes
*This article was written on behalf of the ORAMA study group. The members of the ORAMA study group can be found in the appendix.
REFERENCES
- Locatelli F, Pisoni RL, Combe C, et al. Anaemia in hemodialysis patients of five European countries: Association with morbidity and mortality in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant. 2004; 19: 121–132
- Locatelli F, Aljama P, Barany P, et al. Revised European best practice guidelines for the management of anaemia in patients with chronic renal failure. Nephrol Dial Transplant. 2004; 19: 1–47
- El Nahas M. The global challenge of chronic kidney disease. Kidney Int. 2005; 68: 2918–2929
- Strippoli GF, Tognoni G, Navaneethan SD, Nicolucci A, Craig JC. Hemoglobin targets: We were wrong, time to move on. Lancet. 2007; 369: 346–350
- Pisoni RL, Bragg-Gresham JL, Young EW, et al. Anemia management and outcomes from 12 countries in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2004; 44: 94–111
- Braumann KM, Nonnast-Daniel B, Boning D, Bocker A, Frei U. Improved physical performance after treatment of renal anemia with recombinant human erythropoietin. Nephron. 1991; 58: 129–134
- Kimmel PL, Patel SS. Quality of life in patients with chronic kidney disease: Focus on end-stage renal disease treated with hemodialysis. Semin Nephrol. 2006; 26: 68–79
- National Kidney Foundation II. Clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease in adults. Am J Kidney Dis. 2006; 47: 16–85
- Phrommintikul A, Haas SJ, Elsik M, Krum H. Mortality and target hemoglobin concentrations in anaemic patients with chronic kidney disease treated with erythropoietin: A meta-analysis. Lancet. 2007; 369: 381–388
- Valderrabano F, Horl WH, Jacobs C, et al. European best practice guidelines 1–4: Evaluating anaemia and initiating treatment. Nephrol Dial Transplant. 2000; 15: 8–14
- National Kidney Foundation Kidney Disease Outcome Quality Initiative. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002; 39: 1–249
- Pollock CML. The CARI guidelines. Biochemical and haematological targets guidelines. Hemoglobin. Nephrology (Carlton). 2006; 10: S108–S115
- Horl WH, Vanrenterghem Y, Canaud B, et al. Optimal treatment of renal anaemia (OPTA): Improving the efficacy and efficiency of renal aneamia therapy in hemodialysis patients receiving intravenous epoetin. Nephrol Dial Transplant. 2005; 20: 25–32
- Collins AJ, Roberts TL, St Peter WL, et al. United States Renal Data System assessment of the impact of the National Kidney Foundation—Dialysis Outcomes Quality Initiative guidelines. Am J Kidney Dis. 2002; 39: 784–795
- Jacobs C, Frei D, Perkins AC. Results of the European Survey on Anaemia Management 2003 (ESAM 2003). Current status of anaemia management in dialysis patients, factors affecting epoetin dosage and changes in anaemia management over the last five years. Nephrol Dial Transplant. 2005; 20: 3–24
- Valderrabano F, Horl WH, Macdougall IC, et al. PRE-dialysis survey on anaemia management. Nephrol Dial Transplant. 2003; 18: 89–100
- Hunt DL, Haynes RB, Hanna SE, Smith K. Effects of computer-based clinical decision support systems on physician performance and patient outcomes: A systematic review. JAMA. 1998; 280: 1339–1346
- Grimshaw J, Freemantle N, Wallace S, et al. Developing and implementing clinical practice guidelines. Qual Health Care. 1995; 4: 55–64
- Friedman C, Wyatt J. Evaluation Methods in Medical Informatics. Springer, New York 1997
- EBPG Working Party. European best practice guidelines for the management of anaemia in patients with chronic renal failure. Working Party for European Best Practice Guidelines for the Management of Anaemia in Patients with Chronic Renal Failure. Nephrol Dial Transplant. 1999; 14: 1–50
APPENDIX
ORAMA Steering Committee
Adrian Covic, MD, PhD, Dialysis and Transplantation Centre, Parhon University Hospital, Iasi, Romania
Francesco Locatelli, MD, FRCP, Department of Nephrology and Dialysis, Ospedale A Manzoni, Lecco, Italy
Iain C. Macdougall, MD, FRCP, Renal Unit, King's College Hospital, London, UK
Andrzej Wiecek, MD, PhD, Department of Nephrology, Endocrinology and Metabolic Diseases, Medical
University of Silesia, Katowice, Poland
Principal Investigators
Bulgaria
Peter Shikov, Pazardjik
Dimitar Nikolov, Plovdiv
Boriana Deliyska, Sofia
Dobrin Paskalev, Varna
Pencho Simeonov, Sofia
Croatia
Zarko Belavic, Karlovac
Barbic Jerko, Osijek
Ninoslov Leko, Slavonski Brod
Ivan Bogadi, Varazdin
Germany
Roland E. Winkler, Rostock
Martin Buhl, Berlin
Eckhard Wilbrandt, Heringen
Martin Edinger, Eschwege
Margit Mall, Halle
Christoph Dammerboer, Herzberg
Holger Urzowski, Finsterwalde
Daniela Flender, Sinsheim
Gabriele Kunowski, Heilbronn
Alexander Müller, Weinheim
Hans Anschütz, Groß Gerau
Gerhard Prager, Bad König
Johann-Borwin, Lüth Hannover
Italy
Francesco Locatelli, Lecco
Natale Gaspare De Santo, Napoli
Cosimo Lodeserto, Tarant
Mario Bonomini, Chieti
Gina Meneghel, Dolo
Mauro Ragaiolo, Ascoli Piceno
Giovanni Cancarini, Brescia
Ugo Rotolo, Palermo
Latvia
L. Zepa, Rezekne
G. Saumane-Baza, Valmiera
G. Ritovs, Riga
R. Rozentals, Riga
Poland
Andrezej Rydzewski, Warszawa
Danuta Antczak-Jedrzejczak, Gorzow
Wlodzimierz Ratajewski, Kalisz
Andrzej Wiecek, Katowice
Dorata Frankiewicz, Konin
Olech Mazur, Koszalin
Bogdanowicz Grazyna, Opole
Kosicki Andrezej, Przemysi
Sydor Antoni, Tarnow
Muszytowski Marek, Torun
Romania
Adrian Covic, Iasi
Gabriel Mircescu, Bucharest
Serbia
Marina Mugosa Ratkovic, Podgorica
Steva Pljesa, Zemun
Vidosava Nesic, Belgrade
Nada Dimkovic, Belgrade
Svobodan Curic, Novi Sad
Marina Lazarevic, Kragujevac
Zoran Kovacevic, Belgrade