Abstract
Adult rats submitted to perinatal salt overload presented renin-angiotensin system (RAS) functional disturbances. The RAS contributes to the renal development and renal damage in a 5/6 nephrectomy model. The aim of the present study was to analyze the renal structure and function of offspring from dams that received a high-salt intake during pregnancy and lactation. We also evaluated the influence of the prenatal high-salt intake on the evolution of 5/6 nephrectomy in adult rats. A total of 111 sixty-day-old rat pups from dams that received saline or water during pregnancy and lactation were submitted to 5/6 nephrectomy (nephrectomized) or to a sham operation (sham). The animals were killed 120 days after surgery, and the kidneys were removed for immunohistochemical and histological analysis. Systolic blood pressure (SBP), albuminuria, and glomerular filtration rate (GFR) were evaluated. Increased SBP, albuminuria, and decreased GFR were observed in the rats from dams submitted to high-sodium intake before surgery. However, there was no difference in these parameters between the groups after the 5/6 nephrectomy. The scores for tubulointerstitial lesions and glomerulosclerosis were higher in the rats from the sham saline group compared to the same age control rats, but there was no difference in the histological findings between the groups of nephrectomized rats. In conclusion, our data showed that the high-salt intake during pregnancy and lactation in rats leads to structural changes in the kidney of adult offspring. However, the progression of the renal lesions after 5/6 nephrectomy was similar in both groups.
INTRODUCTION
Several lines of evidence show that the renin-angiotensin system (RAS) participates in renal development.Citation[1–5] Angiotensin II (AII) can act as a growth modulator of several cell types and tissues.Citation[1],Citation[2] Angiotensinogen expression in the rat kidney increases during late pregnancy and peaks during the newborn period, when it reaches considerably higher levels than in the adult. In the rat kidney, renin mRNA is detected from embryonic day 17 and continues to be high during late pregnancy.Citation[3] Renin mRNA levels are approximately 20-fold and 10-fold higher, respectively, at 20 days of embryonic life and in newborns than in adults.Citation[4] The AII receptors are also expressed in a higher amount in neonate rats.Citation[5] It was demonstrated that gene expression for type I AII receptor (AT1), a subtype predominant in the kidney, is up-regulated after decreased sodium intake.Citation[6] Renal expression of the type 2 AII receptor (AT2) has been shown increased during fetal life and decreases after birth.Citation[7] The transient expression of AT2 suggests that AII has a role in kidney development. It also found that the majority of the AII receptors expressed in the mesenchyme of the developing rat embryo is AT2,Citation[7] suggesting that AII is also involved in mesenchymal differentiation through the AT2 receptor.
Several epidemiological and experimental studies have shown that the exposure to an adverse environment in utero appears to program physiology and metabolism permanently, with long-term consequences for the health of the offspring. This phenomenon is termed Programming.Citation[8],Citation[9] Higher blood pressure was observed in newborns from dams submitted to high sodium intake during pregnancy and lactation.Citation[10],Citation[11] It was observed by Silva et al. that adult rats submitted to prenatal salt overload presented RAS functional disturbances.Citation[12] They found that plasma renin activity did not modify in response to high salt intake in the adult rats submitted to a high-salt environment during the prenatal period. The RAS contributes to the renal damage in 5/6 nephrectomy or kidney remnant model.Citation[13],Citation[14] This model is characterized by loss of renal function, proteinuria, hypertension, and histological abnormalities similar to those observed in many human diseases.Citation[13–15] Several structural alterations have been observed in the kidneys of these animals, such as mesangial expansion, glomerulosclerosis, interstitial fibrosis, and mononuclear cellular infiltration.Citation[13–15]
The aim of the present study was to analyze the renal structure and function of rat offspring from dams that received a high salt intake during pregnancy and lactation submitted to 5/6 nephrectomy or a sham operation.
MATERIALS AND METHODS
Animals
Male Wistar (n = 111) rats born from 44 pregnant female weighing ∼180 g were used in this study. All rats were housed in rooms with controlled temperature about 25°C and a 12-hour light/dark cycle. Food and water were supplied ad libitum. All experimental procedures were conducted in accordance with our institutional guidelines.
Experimental Protocol for Pregnant Females
Pregnant females were carefully observed at the end of pregnancy to determine the exact birth date of the pups. During pregnancy and lactation, 22 females were submitted to normal sodium intake, and 22 females received 0.15 mol/L sodium chloride solution instead of water. Urine and plasma samples were collected from pregnant females at the beginning and end of pregnancy using metabolic cages, and 24 hours urine volume was measured. The samples were frozen for subsequent analysis of osmolality, sodium, and potassium. Liquid and food consumption and body weight variation during pregnancy were measured in mothers of both groups. Blood pressure of pregnant rats was measured at the beginning and at the end of pregnancy by the tail-cuff method.
Experimental Protocol for the Thirty- and Sixty-Day-Old Pups
Blood pressure and albuminuria were evaluated in 30- and 60-day-old animals born of dams submitted to high salt intake during pregnancy and lactation and in animals of the same age born of dams submitted to normal sodium intake. Glomerular filtration rate (GFR) was evaluated by inulin clearance in fifteen 30-day-old rats born of dams submitted to high salt intake and in ten same age control rats.
Experimental Protocol for Pups Submitted to 5/6 Nephrectomy or a Sham Operation
Sixty days after birth, pups born from dams submitted to high or normal salt intake during pregnancy and lactation were subjected to 5/6 renal ablation by removal of the right kidney and to infarction of approximately 2/3 of the left kidney by ligation of two or three branches of the left renal artery. The control group (Sham) was submitted to sham operation consisting of laparotomy and manipulation of the renal pedicles. After the surgical procedure, the animals were divided into the following groups:
group S (Sham), which consisted of rats from dams that received normal sodium intake (n = 12);
group SS (Sham Saline), which consisted of rats from dams submitted to high sodium intake (n = 15);
group NE (Nephrectomized), which consisted of nephrectomized rats from dams that received normal sodium intake (n = 45); and
group NES (Nephrectomized Saline), which consisted of nephrectomized rats from dams submitted to high sodium intake (n = 39).
Urine samples were collected to quantify albumin excretion, and the systolic blood pressure was measured by the tail-cuff method, before and after the surgical procedure. The GFR was evaluated by inulin clearance at 120 days after surgery. The animals were killed with excess anesthesia 120 days after surgery and the kidneys removed for immunohistochemical and histological analysis.
Renal Function Studies
The animals were anesthetized with an i.p. injection of 40 mg/kg tionembutal. The body temperature was maintained at 37°C during the experiment. After tracheostomy, the femoral artery and vein were cannulated to collect blood samples and inject fluids. The ureters were cannulated to collect urine. Inulin was administered in a priming dose (8 mg/100 g), followed by a maintenance dose (30 mg/100 g/h). After an approximately 60-minute stabilization period, urine was collected for 1 hour, and blood samples were collected at 30 minutes and 1 hour. These samples were used to assess levels of sodium, potassium, albumin, and inulin. Inulin was measured in plasma and urine samples using the method described by Führ et al.Citation[16] Albumin was quantified in urine samples through electroimmunoassay using a specific antibody against rat albumin.Citation[17] Plasma and urine sodium and potassium were measured by flame photometry (Micronal, model 262, São Paulo, Brazil).
Histological and Immunohistochemical Analysis
The kidneys were perfused through the aorta with PBS (0.15 M NaCl, 0.01 M phosphate buffered, pH 7.4) until they were blanched. The kidneys were then perfused with 4% paraformaldehyde, fixed in the same for two hours, postfixed in Bouin's solution for an additional 4–6 hours, rinsed with 70% ethanol to eliminate picric acid, dehydrated through a graded alcohol series, and embedded in paraffin. Sections of 3 μm of renal tissue from these animals were stained with Masson's trichrome for histological analysis. The glomerular mesangial expansion was evaluated using scores that reflected changes in the extent (0 = 5–25%, 1 = 25–50%, 3 = 50–75%, and 4 = 75–100%), and a mean score by biopsy was calculated. Tubulointerstitial injury was graded according to the system devised by Shih et al.Citation[18]: normal = 0, small focal areas = 0.5, cortical involvement <10% = 1, cortical involvement of 10–25% = 2, cortical involvement of 25–75% = 3, and extensive damage involving more than 75% of the cortex = 4.
The sections were deparaffinized for immunohistochemical studies and incubated for one hour with a monoclonal antibody anti-ED1 (1:1000) at room temperature. This antibody reacts with cytoplasmatic antigen present in macrophages and monocytes (Serotec, Oxford, UK). The reaction product was detected with an avidin-biotin-peroxidase complex (Vector Laboratories, Burlingame, California, USA). The material was counterstained with methyl green, dehydrated, and mounted. Negative controls consisted of replacement of primary antibody with equivalent concentrations of normal mouse IgG. To obtain the numbers of ED1-positive cells in the glomeruli and in the cortical tubulointerstitium, all glomeruli and 30 grid fields from the renal cortical tubulointerstitium measuring 0.245 mm2 each were evaluated, and the mean counts per glomerulus and per cortical tubulointerstitial area per kidney were calculated.Citation[19]
Statistical Analysis
Data related to the histological scores and to albuminuria do not present a normal distribution and were analyzed statistically using the nonparametric Kruskal-Wallis test followed by the Dunn post-test. These results are expressed as median and interquartile (25–75%) ranges. The remaining data were submitted to analysis of variance with Newman-Keuls multiple comparisons test. These results are expressed as mean ± SEM. The level of significance was set at p < 0.05.
RESULTS
Urinary Volume, Blood Pressure, Body Weight Variation, Food Consumption, and Fluid Intake of Pregnant Females
Twenty-four-hour urine volume was higher on pregnant females submitted to salt intake compared to pregnant females from the control group (see ). Liquid intake and body weight variation during pregnancy were 73.15 mL/24 h ± 3.16 and 175.40 g ± 10.29, respectively, on pregnant females submitted to high salt intake, and 52.73 mL/24 h ± 3.24 and 102.90 g ± 18.69, respectively, in pregnant females from the control group (p < 0.05). Taken together, these results may reflect an increase in extracellular fluid volume from dams of experimental group. The systolic blood pressure from pregnant females measured at the beginning and the end of pregnancy was not different in the dams from the experimental group compared to the control group (see ).
Table 1 Systolic blood pressure (SBP) and 24-hour urine volume (V) at the beginning and end of pregnancy of dams submitted to normal sodium intake (control) or increased sodium intake (saline)
Food consumption was not different in dams from the experimental group compared to the control group. Sodium and potassium plasma levels and plasma osmolality did not show any difference between pregnant females from the control and experimental groups (data not shown).
Systolic Blood Pressure, Renal Function, and Albuminuria in Thirty- and Sixty-Day-Old Pups
Thirty- and sixty-day-old rat pups from dams submitted to high sodium intake presented higher systolic blood pressure (SBP) as compared to the same age control rats (see ). Thirty-day-old animals born of dams submitted to increased salt intake during pregnancy and lactation also showed a decrease in GFR (0.67 mL/min/100g ± 0.05) and higher albuminuria (0.29 mg/24 hour) compared to the same age controls (0.80 mL/min/100 g ± 0.07 and 0.15 mg/24 hour, respectively; p < 0.05).
Table 2 Systolic blood pressure (SBP) of thirty- and sixty-day-old rat pups from dams submitted to high-sodium intake (saline group) and same-age control rats before surgery
Systolic Blood Pressure, Renal Function, and Albuminuria of 5/6 Nephrectomized and Sham Operated Rats
Increased SBP was observed in the animals submitted to nephrectomy. However, we found no difference between neither the S and SS groups nor in the NE and NES groups on SBP at 30, 60, and 90 days after surgery (see ).
Table 3 Sytolic blood pressure (SBP, mmHg) of rats subjected to 5/6 nephrectomy (NE) or a sham operation (S), born from dams submitted to high (SS, NES) or normal (S, NE) sodium intake, 30, 60 and 90 days after the operation
Higher albuminuria and a decrease in GFR were observed in both groups of animals with 5/6 nephrectomy, compared to the rats from sham groups. However, there is no difference between neither the S and SS groups nor the NE and NES groups at 120 days after surgery in these parameters of renal function (see ).
Table 4 Glomerular filtration rate (GFR, ml/min/100 g) and albumin urinary excretion (albuminuria, mg/24 h) of rats from dams submitted to high-sodium intake (SS, NES) and same-age controls (S) at 120 days after nephrectomy (NE, NES) or to sham operation (S, SS)
Histological and Immunohistochemical Analysis of 5/6 Nephrectomized and Sham Operated Rats
The histological analysis showed the presence of higher scores for glomerulosclerosis and tubulointerstitial lesions in renal cortex from the rats of the SS group compared to the S group at 120 days after surgery. The 5/6 nephrectomized rats presented intense histological damage compared to sham rats, characterized by glomerulosclerosis, interstitial fibrosis, inflammatory infiltrate, tubular lumen dilation and atrophy, but there was no difference between the animals of NE and NES groups (see and ).
Figure 1. Masson's trichrome stained histological sections of renal cortex of rats from dams submitted to high-sodium intake (B and D) and of the same age control rats (A and C) at 120 days after nephrectomy (C and D) or a sham operation (A and B), × 280.
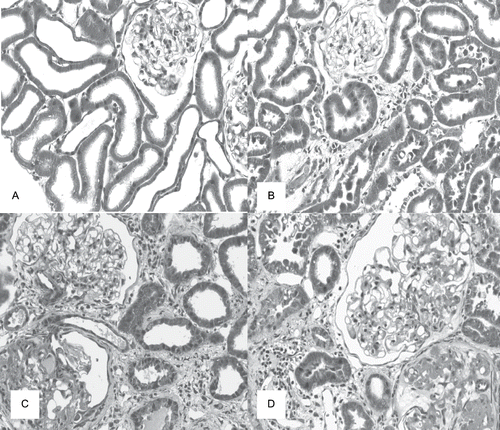
Table 5 Glomerulosclerosis (GS) and tubulointerstitial lesions (TIL) scores of rats from dams submitted to high-sodium intake (SS and NES) and of the same age controls rats (S) at 120 days after nephrectomy (NE) or sham operation (S)
A higher number of ED1-positive cells (macrophages/monocytes) was found in glomeruli and tubulointerstitium from the cortex of rats from SS group compared to S group. The number of ED1-positive cells was increased in glomeruli and tubulointerstitium from the renal cortex of both groups of 5/6 nephrectomized rats, compared to sham rats (see and ).
Figure 2. Immunolocalization of ED1 (macrophages/monocytes) in renal cortex of rats from dams submitted to high-sodium intake (B and D) and of the same age control rats (A and C) at 120 days after nephrectomy (C and D) or a sham operation (A and B), × 280.
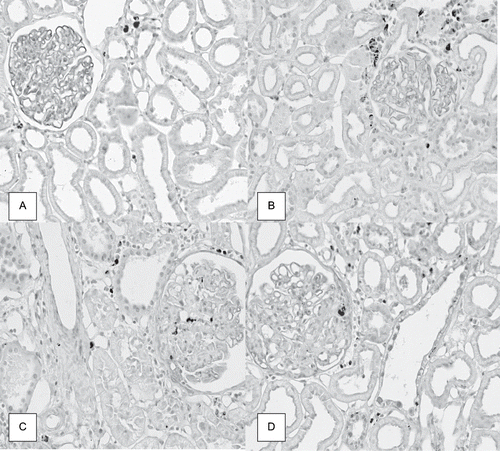
Table 6 Number of ED1-positive cells per glomerulus or per area of renal cortical tubulointerstitium, measuring 0.245 mm2 each, of rats from dams submitted to high-sodium intake (SS and NES) and of the same age controls rats (S) at 120 days after nephrectomy (NE) or sham operation (S)
DISCUSSION
Our data show that the 30-day-old pups from dams submitted to high sodium intake during pregnancy and lactation presented increased SBP and albuminuria and decreased GFR compared to the same age control rats. The scores for tubulointerstitial lesions and glomerulosclerosis were also higher in the rats from the Sham Saline group compared to control rats 120 days after a sham operation. These data demonstrate that disturbances occurring during intrauterine life can induce persistent alterations in the biology of the pups in adult life.
In previous studies, we observed that increased sodium intake during pregnancy provokes reductions of α-SM-actin, fibronectin, PCNA (proliferating cell nuclear antigen), and AII expression in the renal cortex from neonate rats, interfering with renal development and renal function during adult life.Citation[11] We also found that there was a temporal association between the decrease of AII expression and the reduction of α-SM-actin, PCNA, and fibronectin expression in renal cortex, suggesting a possible relationship between these findings.
Twenty-four-hour urine volume and liquid intake were higher in the pregnant females submitted to high salt intake compared to the pregnant females from the control group. The body weight variation was also higher in the pregnant females from the experimental group compared to pregnant females from the control group. Taken together, these results may reflect an increase of extracellular fluid volume in dams from the experimental group that can lead to a decrease of the RAS activity. The alteration in RAS activity can be also happening in the newborn rats. Newborn rats from dams submitted to high salt intake presented a decrease of the number of AII-positive cells in renal cortex at 1 and 7 days of age compared to the same age control rats.Citation[11] It has been observed that local AII levels may depend on in situ synthesis, an uptake of the peptides from the circulation, or a combination of the two.Citation[20–22] AII has important effects on the cell growth and extracellular matrix production.Citation[23–25]
Formation of the extracellular matrix represents a key event in kidney cell differentiation.Citation[25] The mRNA for renin in the rat kidney and angiotensinogen expression during gestation are higher in neonates than in adult rats.Citation[3],Citation[4] The AII receptors are also expressed in a higher amount in neonate rats.Citation[5] Guron and Friberg observed that an intact RAS is a prerequisite for normal renal development.Citation[26] Gribouval et al. reported that mutations in genes in the RAS are associated with autosomal recessive renal tubular dysgenesis.Citation[27]
The data of the present study also show that the 30-and 60-day-old rats from dams exposed to increased sodium ingestion also presented higher SBP compared to the same age control rats. It has been previously reported that a prenatal high salt diet increases blood pressure and salt retention in spontaneously hypertensive rats.Citation[28] Hazon et al. and Silva et al. found that adult offspring from high salt intake dams presented increased blood pressure,Citation[10],Citation[12] increased renal AII content, and an absence of plasma renin changes in responses to high salt consumption.Citation[12] We observed a decrease in the expression of AT1 receptor in one-day-old rats born from dams submitted to high salt intake compared to control of same age.Citation[11] Therefore, the higher blood pressure observed in 30- and 60-day animals of the saline group may be a consequence of RAS functional changes that may occur in offspring from dams with high salt intake during pregnancy and lactation. However, the present study showed that after 5/6 nephrectomy, the changes in SBP and in the renal function and structure were not different in rats from dams submitted to high salt intake during pregnancy and lactation compared to 5/6 nephrectomized rats from NE group, probably because the 5/6 nephrectomy is a very intense insult and the local renal regulation of AII production involves mechanisms other than the systemic.
A higher level of proteinuria was observed in both groups of nephrectomized rats, but we did not observe any difference between both groups. Proteinuria can induce an increase in renal AII and inflammation.Citation[29] The number of ED1-positive cells (macrophages/monocytes) was increased in the glomeruli and tubulointerstium of renal cortex from the rats of both groups with 5/6 nephrectomy. These cells can produce AII,Citation[30],Citation[31] which has an important role in the renal lesion progression in 5/6 nephrectomized rats.Citation[13],Citation[14] In addition to its effect on renal and systemic hemodynamics, AII also contributes to the inflammatory process and fibrosis observed in these animals.Citation[32] These effects might be mediated at least in part by the activation of the NF-κB (nuclear factor κB) pathway.Citation[33]
There are also other factors present in these animals with 5/6 nephrectomy that can also induce the activation of NF-κB, such as oxidative stress and proteinuria.Citation[29],Citation[34] NF-κB is an important transcription factor that induces the transcriptional activation of target genes related to the inflammatory process (cytokines, growth factors and adhesion molecules, as well as macrophage and monocyte chemotactic factors), provoking damage in the kidneys.Citation[33],Citation[34] Macrophages can release fibrogenic peptides, such as TGF-β (transforming growth factor β), interleukin-1, endothelin, AII and radical oxygen species, contributing to the inflammatory process and fibrosis.Citation[35]
In conclusion, our data showed that the high salt intake during pregnancy and lactation in rats leads to structural changes in the kidney of adult offspring. However, the progression of the renal lesions was similar in both groups of 5/6 nephrectomized rats. This could be explained by the fact that the local renal regulation of AII production involves different mechanisms than the systemic, and because the 5/6 nephrectomy is a very aggressive insult.
ACKNOWLEDGMENTS
The authors thank Erika Delloiagono, Adriana L. G. de Almeida, and Rubens Fernando de Melo for expert technical assistance. Evelyn Cristina Santana Marin was a recipient of a Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, DF, Brazil, fellowship, and Dr. Roberto Silva Costa and Dr. Terezila Machado Coimbra are recipients of Conselho Nacional de Desenvolvimento Científico e Tecnológico, DF, Brazil, fellowships.
REFERENCES
- Bagby SP, Kirk EA, Mitchell LH, O´Reilly MM, Holden WE, Stenberg PE, Bakke AG. Proliferative synergy of angiotensin II and EGF in porcine aortic vascular smooth muscle cells. Am J Physiol. 1993; 265: F239–F249
- Fernandez LA, Twickler J, Mead A. Neovascularization produced by angiotensin II. J Lab Clin Med. 1985; 105: 141–145
- Gomez RA. Molecular biology of components of the renin-angiotensin system during development. Pediatr Nephrol. 1990; 4: 421–423
- Gomez RA, Lynch KR, Sturgill BC, Elwood JP, Chevalier RL, Carey RM, Peach MJ. Distribution of renin mRNA and its protein in the developing kidney. Am J Physiol. 1989; 257: F850–F858
- Millan MA, Carvallo P, Izumi S, Zemel S, Catt KJ, Aguilera G. Novel sites of expression of functional angiotensin II receptors in the late gestation fetus. Science. 1989; 244: 1340–1342
- Tufro-McReddie A, Harrison JK, Everett AD, Gomez RA. Ontogeny of type 1 angiotensin II receptor gene expression in the rat. J Clin Invest. 1993; 91: 530–537
- Grady EF, Sechi LA, Griffin CA, Schambelan M, Kalinyak JE. Expression of AT2 receptors in the development rat fetus. J Clin Invest. 1991; 88: 921–933
- Barker DG. Intrauterine programming of adult disease. Mol Med Today. 1995; 1: 418–423
- Vehaskari VM, Aviles DH, Manning J. Prenatal programming of adult hypertension in the rat. Kidney Int. 2001 Jan; 59: 238–245
- Hazon N, Parker C, Leonard R, Henderson IW. Influence of an enriched dietary sodium chloride regime during gestation and suckling and post-natally on the ontogeny of hypertension in the rat. J Hypertens. 1988; 6: 517–524
- Balbi APC, Costa RC, Coimbra TM. Postnatal renal development of rats from mothers that received increased sodium intake. Pediatr Nephrol. 2004; 19: 1212–1218
- Silva AA, Noronha IL, Oliveira IB, Malheiros DM, Heinemann JC. Renin-angiotensin system function and blood pressure in adult rats after perinatal salt overload. Nutr Metab Cardiovasc Dis. 2003; 13: 133–139
- Monteiro de Freitas AS, Coimbra TM, Costa RS, Baroni EA. Urinary transforming growth factor-beta excretion (TG-beta) and renal production of TGF-beta in subtotal renal ablation: Effect of enalapril and nifelipine. Nephron. 1998; 78: 302–309
- Gretz N, Waldherr R, Strauch M. The remnant kidney model. In: eds. Rat Model of Chronic Renal Failure, N Gretz, M Strauch. Karger, Basel 1993; 1–28
- Floege J, Alpers CE, Burns MW, Pritzl P, Gordon K, Couser WG, Johnson RJ. Glomerular cells, extracellular matrix accumulation, and the development of glomerulosclerosis in the remnant kidney model. Lab Invest. 1992; 66: 485–497
- Führ Y, Kaczmarczk Y, Kruttgen GD. Eine einfache colorimetrische methode zur inulin bestimmung fur nieren clearance-untersuchungen bei stoffwechselgesunden und diabetikern. Klin Wochnschr. 1955; 33: 729–730
- Laurell CB. Electroimmunoassay. Scand J Clin Lab Invest. 1972; 124(1)21–23
- Shih W, Hines WH, Nielasen EG. Effects of cyclosporin A on the development of incubated with the antibody immune-mediated interstitial nephritis. Kidney Int. 1988; 33: 1113–1118
- Coimbra TM, Janssen U, Gröne HJ, Ostendorf T, Kunter U, Schmidt H, Brabant G, Floege J. Early events leading to renal injury in obese Zucker (fatty) rats with type II diabetes. Kidney Int. 2000; 57: 167–182
- Van Kats JP, Schalekamp MA, Verdouw PD, Duncker VJ, Danser H. Intrarenal angiotensin II: Interstitial and cellular levels and site production. Kidney Int. 2001; 60: 2311–2317
- Ingert C, Grima M, Coquard C, Barthelmebs M, Imbs JL. Contribution of angiotensin II internalization to intrarenal angiotensin II levels in rats. Am J Physiol Renal. 2002; 283: F1003–F1010
- Zhuo JL, Imig JD, Hammond TG, Orengo S, Benes E, Navar LG. Ang II accumulation in rat renal endossomes during Ang II-induced hypertension: role of AT 1 receptor. Hypertension. 2002; 39: 116–121
- Hsuch WA, Do YS, Anderson PW, Law RE. Angiotensin II in cell growth and matrix production. Adv Exp Med Biol. 1995; 377: 217–223
- McCausland JE, Ryan GB, Alcorn D. Angiotensin converting enzyme inhibition in the postnatal rat results in decreased cell proliferation in the renal outer medulla. Clin Exp Pharmacol Physiol. 1996; 23: 552–554
- Thiery JP, Duband JL, Dufour S, Savagner P, Imhof BA. Role of fibronectin in embryogenesis. In: ed. Biology of Extracellular Matrix: Fibronectin, DF Mosher. Academic Press, San Diego, Calif 1989; 181–212
- Guron G, Friberg P. An intact renin angiotensin system is a prerequisite for normal renal development. J Hypertension. 2000; 18: 123–137
- Gribouval O, Antignac C, Gubler MC. Mutations in genes in the renin-angiotensin system and renal tubular dysgenesis. Med Sci (Paris). 2006; 22: 246–248
- Nicolantonio RD, Spargo S, Morgan TO. Prenatal high salt diet increases blood pressure and salt retention in spontaneously hypertensive rat. Clin Exp Pharmacol Physiol. 1987; 14: 233–235
- Takase O, Marumo T, Imai N, Hirahashi J, Takayanagi A, Hishikawa K, Hayshi M, Shimizu N, Fujita T. NF-κB-dependent increase intrarenal angiotensin II by proteinuria. Kidney Int. 2005; 68: 464–473
- Okamura A, Rakugi H, Ohishi M, Yagitani Y, Takiuchi S, Moriguchi K, Fennessy PA, Higaki J, Ogihara T. Upregulation of renin-angiotensin system during differentiation of monocytes to macrophages. J Hypertens. 1999; 17: 537–545
- Potter DD, Sobey CG, Tompkins PK, Rossen JD, Heistad DD. Evidence that macrophages in atherosclerotic lesions contain angiotensin II. Circulation. 1998; 98: 800–807
- Noronha IL, Fujihara CK, Zatz R. The inflammatory component in progressive renal disease—are interventions possible?. Nephrol Dial Transplant. 2002; 17: 363–368
- Costa JCR, Costa RS, da Silva CA, Coimbra TM. Enalapril reduces the expression of nuclear factor-kappaB and c-Jun N-terminal kinase in the renal cortices of five-sixths-nephrectomized rats. Am J Nephrol. 2006; 26: 281–286
- Hill CS, Treisman R. Transcriptional regulation by extracellular signals: Mechanisms and specificity. Cell. 1995; 80: 199–211
- Eardley SK, Cockwell P. Macrophages and progressive tubulointerstitial disease. Kidney Int. 2005; 68: 437–455