Abstract
The study evaluates the effect of genistein, a soy isoflavone, on insulin sensitivity and renal functional and structural injury in rats rendered insulin-resistant by feeding on a high-fructose diet for 60 days. Fructose-fed animals (60 g /100 g) displayed insulin resistance as indicated by the measures of insulin sensitivity [insulin sensitivity index (ISI0,120), quantitative insulin check index (QUICKI), and homeostatic model assessment (HOMA)]. Alterations in body weight, kidney weight, urine volume, plasma, and urine electrolytes accompanied by significant increases in plasma and urinary levels of urea, uric acid, creatinine, total protein, and albumin were observed in fructose-fed rats. Oxidative stress in kidney was noted by an elevation in lipid peroxides and a decline in glutathione (GSH). Insulin sensitivity and renal function were improved in fructose-fed rats administered genistein. Histological changes such as fatty infiltration and thickening of glomeruli observed in fructose-fed rats were also ameliorated when genistein was co-administered. The study shows that genistein improves insulin sensitivity and kidney function in a dietary model of insulin resistance. We suggest that genistein may have benefits for patients suffering from kidney disease associated with insulin resistance.
INTRODUCTION
A well-known rodent model of insulin resistance utilizes the administration of high dosage of fructose (60g/100g) as the sole source of carbohydrate in the diet. High dosage of fructose has been documented to induce insulin resistance, glucose intolerance, hyperglycemia, hypertriglyceridemia, and hypertension in rats and is said to parallel the human multimetabolic syndrome.Citation[1–3] Studies from our laboratory have shown that the administration of fructose facilitates renal damage associated with poor antioxidant potential and oxidative stress.Citation[4] Renal changes including renal hypertrophy, arteriolopathy, glomerular hypertension, and cortical vasoconstriction have been reported in fructose overloaded rats.Citation[5]
Genistein, a phytochemical with isoflavone structure, is found in a wide variety of plant-derived foods, in particular, soybeans. Soybean extract has been shown to have protective effects against cardiovascular risk, atherosclerosis, and type 2 diabetes that are attributed to its antioxidant activity.Citation[6–8] The other biological activities of genistein that are reported are the inhibition of tumor cell proliferation, induction of tumor cell differentiation, cell cycle arrest, and apoptosis.Citation[9]
Park et al.Citation[10] have noted that genistein exerts an antidiabetic effect in type 2 diabetic subjects by improving glucose and lipid metabolism. A study has demonstrated the beneficial effects soy isoflavones on kidney damage induced by nephrotoxin.Citation[11] However, there is a lack of data that directly relate the improvement of insulin action and renal function by genistein in the insulin-resistant state. Consequently, the present study was conducted to examine the impact of genistein on changes in insulin sensitivity and renal function and structure in high-fructose diet- induced insulin-resistant rats.
MATERIALS AND METHODS
Chemicals
Genistein (Sigma Chemicals Company, St. Louis, Missouri, USA) was obtained as a generous gift from Dr. T. Szkudelski from the Department of Animal Physiology and Biochemistry, August Cieszkowski University of Agriculture, Poland. Kits for glucose and insulin assay were obtained from Agappe Diagnostics Pvt, Ltd., Kerala, India, and Accubind Microwells, Monobind Inc., Lake Forest, California, USA, respectively. All other chemicals and solvents required for the study were purchased from SISCO Research Laboratories Pvt. Ltd., Mumbai, India.
Animals
Adult male Wistar rats (body weight, 150–160 g) were obtained from the Central Animal House, Rajah Muthiah Medical College, Annamalai University. They were housed in a well-ventilated animal room under controlled conditions on a 12 h light/12 h dark cycle. Animals received a standard pellet diet (Karnataka State Agro Corporation Ltd., Agro feeds division, Bangalore, India) and water ad libitum. The experimental procedures were cleared and approved by the Institutional Ethical Committee of Animal Care, Rajah Muthiah Medical College, Annamalai University.
Experimental Design
The animals were divided into six groups of six rats each and were maintained as follows:
group 1 (CON): control animals received the control diet containing starch as the carbohydrate source.
group 2 (FRU): fructose-fed animals received the high-fructose diet.
group 3 (FRU+GEN): fructose-fed animals received genistein (1 mg/Kg/day in 0.5 ml dimethyl sulfoxide, i.p) daily from the 16th day of the experimental period.
group 4 (CON+GEN): control animals received genistein (1 mg/Kg/day in 0.5 ml dimethyl sulfoxide, i.p) daily from the 16th day of the experimental period.
The control and test diet were prepared fresh every day, the composition of which are given in . The animals were maintained in their respective groups for an experimental period of 60 days, and body weights were recorded at regular intervals. The animals were kept in individual metabolic cages, and 24 hr urine samples were collected in sealed beakers that contained 0.2 mL of 10 N hydrochloric acid (HCl). Urine volume was measured and then used for the determination of variables.
Table 1 Composition of diet (g/100 g)
Insulin Sensitivity Assessment
On day 59, an oral glucose tolerance test (OGTT) was performed. For this test, a set of six rats in each group were fasted overnight, and blood samples were collected by sinoocular puncture at 0 and 120 minutes after the administration of glucose (2 g/kg). Glucose and insulin were assayed in the plasma samples. OGTT curves were drawn by plotting blood glucose (mg/dL) level against time (min). The integrated area under curve (AUC) for glucose and insulin were calculated and expressed as mg/mL/min for AUCglucose and μU/mL/min for AUCinsulin using Graph Pad Prism Version 5.1.
Whole body insulin sensitivity was assessed by measuring the insulin sensitivity index (ISI0,120),Citation[12] homeostatic model assessment (HOMA),Citation[13] and quantitative insulin check index (QUICKI)Citation[14] values. The formulae used are given below:
where MCR (the metabolic clearance rate) is
and where MPG (mean plasma glucose) is the mean of 0 and 120 min glucose values, MSI (mean serum insulin, mU/I) is calculated as the mean of the 0 and 120 min insulin values, and
Measurement of Creatinine and Urea Clearance
Urea and creatinine clearance were determined by measuring the plasma and urinary levels of urea and creatinine and calculated from the standard equation
where U is concentration of the substance in urine, V is the ml of urine excreted per minute, and P represents concentration of the substance in plasma. The rates were expressed in mL/min/100g body weight.
On day 60, the rats were fasted overnight and blood was collected by sino-ocular puncture. Blood was processed for plasma separation and used for biochemical assays. The animals were sacrificed by cervical dislocation after anaesthesia (ketamine hydrochloride 30 mg/kg i.m). The abdomen was cut opened and kidneys were dissected, washed in ice cold saline, and weighed.
Biochemical Analysis
Portions of renal tissues were cut and homogenized in 0.1 M Tris HCl buffer, pH 7.4 for the assay of thiobarbituric acid reactive substances (TBARS) as described by the method of Niehaus and Samuelson.Citation[15] GSH was measured by the method of Ellman.Citation[16] Plasma and urine concentrations of urea, uric acid, creatinine, and protein were measured in an automated analyzer (Technician-RA-XT Boehringer, Germany). Urine albumin was determined using a kit obtained from Bayer diagnostics, Germany. Sodium and potassium were measured with Easylyte plus-analyser (Hitachi, Japan).
Histopathology Examination
After dissection of the animal, one entire kidney was placed in 10% neutral formalin solution for fixation, embedded in paraffin wax, and stained with hematoxylin and eosin. Sections were examined under bright field microscope.
Statistical Analysis
Values are expressed as means ± SD. Data within the groups are analyzed using one-way analysis of variance followed by Duncan's multiple range test. A value of p < .05 was considered statistically significant.
RESULTS
Changes in body weight, kidney weight, and urine volume during the experimental period are shown in . Body weight, kidney weight, and urine volume were significantly increased in rats fed with high-fructose for 60 days. In the other groups, the values were normal.
Table 2 Changes in body weight, kidney weight, and urine volume of experimental animals
The levels of plasma glucose and insulin and the values of HOMA, QUICKI, and ISI0, 120 in experimental animals are given in . At the end of 60 days, fasting glucose and insulin levels were higher in fructose diet-fed rats. Treatment with genistein reduced the level of plasma glucose and insulin to near normal. Insulin resistance was observed as indicated by a higher HOMA values and lower QUICKI and ISI0, 120 values as compared to control (see ). Rats treated with genistein restored these values and improved insulin sensitivity.
Table 3 Plasma glucose, insulin, and insulin sensitivity indices of experimental animals
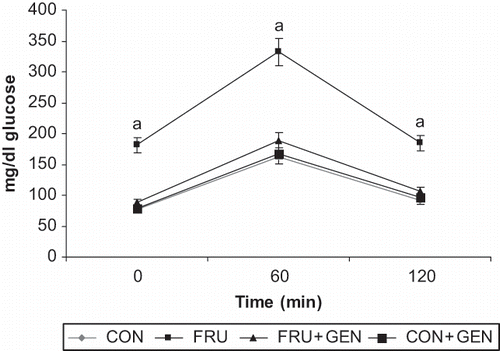
gives the results of the OGTT in experimental animals. The mean fasting glucose level was higher in the fructose-fed rats as compared to control rats. Significant elevations were observed in the glucose level at 60 and 120 min after the glucose load in fructose-fed rats. On the other hand, fasting glucose concentration and the response to glucose load were normal and significantly lower in fructose-fed rats treated with genistein than the untreated fructose-fed rats. Area under the curve (AUCglu-cose mg/mL/min) were CON, 147.25 ± 10.23; FRU, 309.01 ± 23.46; FRU + GEN, 171.97 ± 12.06; and CON + GEN, 149.48 ± 07.62. Area under the curve (AUCinsulin μU/mL/min) were CON, 76.66 ± 5.34; FRU, 147.17 ± 08.74; FRU + GEN, 87.97 ± 6.24; and CON + GEN, 78.57 ± 5.78.
Figure 1. Oral glucose tolerance test curves of experimental animals. Values are means ± SD (n = 6). CON: control rats; FRU: fructose-fed rats; FRU + GEN: fructose-fed rats treated with genistein (1mg/kg b.w); CON + GEN: control rats treated with genistein (1mg/kg b.w). asignificant at p < 0.05 compared to CON ANOVA followed by DMRT.
The levels of plasma urea, uric acid, creatinine, total protein, and albumin in experimental animals are given in . The levels of urea, uric acid, and creatinine were increased, and the level of total protein and albumin were decreased in rats fed high-fructose diet for 60 days. Treatment with genistein restored levels that were comparable to control rats.
Table 4 Levels of urea, uric acid, creatinine, total protein, and albumin in plasma of experimental rats
The levels of urinary urea, uric acid, creatinine, and albumin in experimental animals are shown in . The urinary level of urea, uric acid, and creatinine were significantly decreased, while the urinary albumin level was increased in rats fed with high-fructose diet as compared to control. Treatment with genistein restored these levels as well.
Table 5 Urinary urea, uric acid, creatinine, and albumin of experimental animals
The levels of electrolytes sodium and potassium in plasma and urine of experimental animals are shown in . Rats fed a high-fructose diet for 60 days showed higher levels of sodium in plasma than control animals, while the level of potassium is lower in fructose-fed animals. The urinary levels of both sodium and potassium were significantly increased in rats fed with high-fructose diet. Treatment with genistein to these rats significantly normalized the levels to near-normal.
Table 6 Levels of sodium and potassium in plasma (mEq/L) and urine (mmol/L/day) of experimental animals
Urea and creatinine clearance rates of experimental animals are given in . At the end of 60 days, decreased urea and creatinine clearance was observed in rats fed a high-fructose diet. Rats treated with genistein showed improved glomerular function as indicated by the clearance rates, which were comparable to normal rats.
Table 7 Urea and creatinine clearance rate (ml/min) in experimental animals
The levels of TBARS, a lipid peroxidation index, and GSH, a non-enzymic antioxidant, in experimental rats are shown in . Significant alterations in both were observed in rats fed high-fructose-diet. Treatment with genistein restored the levels to near-normal.
Table 8 Levels of thiobarbituric reactive substances (TBARS) and reduced glutathione (GSH) in kidney of experimental rats
DMSO administered at 12.5–50% concentrations did not produce any significant change in biochemical parameters in normal animals.Citation[17] In our study, control animals treated with genistein in DMSO also showed no appreciable changes, indicating that genistein and DMSO had no effect in control animals.
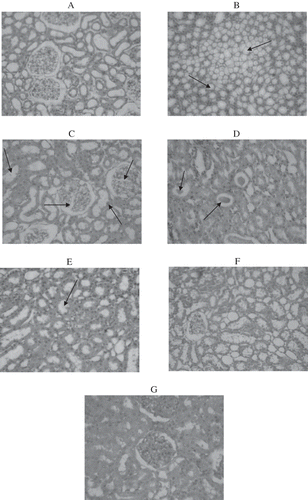
The results of the histopathological study of the kidneys are shown in . shows the histological section of a control rat fed a starch diet, which exhibits normal glomeruli and tubules. There is no fatty infiltration in renal cells. represent the kidney sections from fructose-fed rats, which reveal fatty infiltration in parenchyma (see ) and segmented glomerular nephritis (see ). The tubules show fat accumulation and are filled with hyaline casts. Inflammatory cell infiltrate in the parenchyma is also seen (see and ). The severity of the renal parenchymal injury induced by fructose is mitigated by genistein (see ), and there is a reduction in segmental sclerosis in the glomeruli in genistein-treated animals. Genistein supplementation to control rat (CON + GEN) has no deleterious effects on renal histology (see ).
Figure 2. Histopathology of kidney using hematoxylin and eosin (A–G; H&E × 20). Control rats showed normal glomeruli and tubules (). represent kidney section of high-fructose-fed rats. shows tubules having fatty infiltration. shows segmented glomerulo nephritis, with the tubules showing fat accumulation. shows hyaline casts in tubules. shows fatty infiltration in parenchyma cells, and represents the kidney section of fructose-fed rats treated with genistein, showing cloudy swelling and mild fat accumulation in tubules. represents the kidney section of normal rats treated with genistein in which the glomeruli show mild congestion.
DISCUSSION
The chronic administration of fructose for two months caused insulin resistance and renal changes such as hypertrophy, dysfunction, histological alterations, and oxidative injury. Genistein administration improved insulin sensitivity and decreased oxidative stress and the severity of the glomerular and tubulo interstitial damage.
Renal hypertrophy and glomerular hyperfiltration are the early events in the development of irreversible renal damages.Citation[18] Creatinine clearance continues to be a measure of glomerular filtration in animals subjected to experimental nephrotoxicity and renal failure, and glomerular filtration rate (GFR) is the fundamental parameter of kidney function. In the present study, high-fructose feeding was found to be associated with renal hypertrophy and glomerular hyperfiltration. These changes could be attributed to increased glomerular volume and capillary surface area.Citation[19] Changes in filtration surface area and increased plasma creatinine are observed in other diabetic rat models.Citation[20]
Hyperuricemia is a component of the metabolic syndrome and is implicated in renal disease. Mild hyperuricemia induces intrarenal crystal formation, systemic hypertension, renal vasoconstriction, glomerular hypertension, and hypertrophy and tubulointerstitial injury.Citation[21] The pathophysiological mechanisms by which the uric acid causes these conditions involves an inhibition of endothelial nitric oxide bioavailability,Citation[22] activation of the rennin-angiotensin system,Citation[23] and the direct actions on endothelial cells and vascular smooth muscle cells.Citation[24] Fructose intake may raise uric acid levels by the activation of fructokinase reaction in the liver, leading to ATP depletion, the stimulation of AMP deaminase, and an increase in the degradation of nucleotides to uric acid.Citation[25] Fructose administration has been shown to be associated with endothelial dysfunction and oxidative stress, both of which could contribute to glomerulosclerosis.Citation[26] Conversely, a rise in uric acid may decrease endothelial function in adipocytes that may promote insulin resistance. Uric acid is suggested to play a causal role in fructose-induced metabolic syndrome.Citation[27]
Fructose-fed rats showed sodium retention and depletion of potassium levels. It has been reported that fructose acts as a potent antinatriuretic agentCitation[3] and decreases aldosterone levels. This could be responsible for sodium retention in fructose-fed rats. Decreased excretion of potassium in urine may be attributed to the antikaliuretic effect of fructose feeding,Citation[28] and decreased plasma potassium levels in fructose-fed rats could be due to the shifts of potassium into the cells from extracellular fluid.
The importance of reactive oxygen species (ROS) in renal damage comes from studies that show a protective effect of free radical scavengers on kidney function. The administration of antioxidants reduced the renal accumulation of lipid peroxidation products during nephrotoxicity. ROS have been shown to be involved in the acute reduction in GFR and proximal tubular damage in bilateral renal artery occlusion and in puromycin-induced nephropathy.Citation[29]
Enhanced lipid peroxidation in fructose-fed rats could be associated with high circulating glucose, which enhances free radicals production from glucose autooxidation and protein glycation. The amelioration of renal damage and oxidative stress in this model of diabetic, proteinuric renal disease suggest that genistein's beneficial effect is mediated via its antioxidant capabilities, which prevent the damage caused by ROS. GSH, a peptide with a free thiol group, directly reacts with ROS and plays a crucial role in coordinating the antioxidant defense processes. An increase in renal GSH content in genistein-treated rats in comparison to untreated rats demonstrates the antioxidant effects of genistein. Genistein decreased TBARS and increased GSH level in mice with fatty liver and obesity induced by a high-fat diet.Citation[30] The amelioration of oxidative stress observed in the study is in line with previous reports, which show that genistein decreases oxidative stress and NF-κB activation in murine macrophages.Citation[31] The ability of genistein to reduce ROS formation and suppress oxidative DNA damage have been established.Citation[32] Genistein has been shown to increase the activities of antioxidant enzymes.Citation[33]
The exact mechanism by which fructose consumption might accelerate renal damage remains obscure, but studies suggest a number of associated factors and causes that are triggered by fructose. These include a lipogenic effect, release of inflammatory cytokines, endothelial dysfunction, and oxidative stress, which are interrelated with one another.Citation[34] Further, hyperinsulinemia itself has a direct role in the pathogenesis of renal injury as a consequence of stimulating sympathetic nervous system and renin-angiotensin aldosterone system,Citation[35] and we observed a diminution of insulin levels in genistein-treated rats.
Insulin resistance in the fructose-fed rat model has been attributed to a low level of insulin-stimulated glucose oxidation due to modifications in the post-receptor cascade of insulin action. It is possible that genistein may have effects on the insulin-signaling cascade and post-receptor events. Genistein administration improved insulin sensitivity possibly by restoring GSH levels, as GSH is required for insulin receptor gene activation.Citation[36]
A recent rise in fructose consumption could account for the increase in the prevalence of diabetic renal disease, as fructose intake correlates well with the epidemics of metabolic syndrome. Our data provide evidence for the insulin sensitivity effects of genistein and for its protective effect on fructose-induced kidney injury. We suggest that this isoflavone exerts its effect by acting as an antioxidant and thus could be benefit in the therapy of kidney diseases associated with insulin resistance.
ACKNOWLEDGMENT
The financial support in the form of a Senior Research Fellowship to Mr. N. Palanisamy from the Indian Council of Medical Research (ICMR), New Delhi, is gratefully acknowledged. The author is thankful to Dr. T. Szkudelski, Department of Animal Physiology and Biochemistry, August Cieszkowski University of Agriculture, Poland, for providing genistein.
REFERENCES
- Reaven GM. Banting lecture. Role of insulin resistance in human disease. Diabetes. 2001; 37: 1595–1607
- Elliott SS, Keim NL, Stern JS, Teff K, Havel PJ. Fructose, weight gain, and the insulin resistance syndrome. Am J Clin Nutr. 2002; 76: 911–922
- Hwang IS, Ho H, Hoffman BB, Reaven GM. Fructose-induced insulin resistance and hypertension in rats. Hypertension. 1987; 10: 512–516
- Rajasekar P, Viswanathan P, Anuradha CV. Renoprotective action of L-carnitine in fructose-induced metabolic syndrome. Diabetes Obesity and Metabolism. 2007; 10: 171–180
- Sa´nchez-Lozada LG, Tapia E, Jimenez A, Bautista P, Cristobel M, Nepomuceno T. Fructose-induced metabolic syndrome is associated with glomerular hypertension and renal microvascular damage in rats. Am J Physiol Renal Physiol. 2007; 292: F423–F429
- Azadbakht L, Shakerhosseini R, Jamshidian M, Mehrabi Y, Esmaill-Zadeh A. Beneficiary effect of dietary soy protein on lowering plasma levels of lipid and improving kidney function in type II diabetes with nephropathy. Eur J Clin Nutr. 2003; 57: 1292–1294
- Vedavanam K, Srijayanta S, O'Reilly J, Ranam A, Wiseman H. Antioxidant action and potential antidiabetic properties of an isoflavonoid-containing soybean phytochemical extract (SPE). Phytother Res. 1999; 13: 601–608
- Lee DS, Lee SH. Genistein, a soy isoflavone, is a potent alpha-glucosidase inhibitor. FEBS Lett. 2001; 501: 84–86
- Li Y, Ellis KL, Ali S, El-Rayes BF, Nedeljkovic-Kurepa A, Kucuk O, Philip PA, Sarkar FH. Apoptosis-inducing effect of chemotherapeutic agents is potentiated by soy isoflavone genistein, a natural inhibitor of NF-kappa B in B×PC-3 pancreatic cancer cell line. Pancreas. 2004; 28(4)e90–e95
- Park SA, Choi MS, Cho SY, Seo JS, Jung UJ, Kim MJ. Genistein and daidzein modulate hepatic glucose and lipid regulating enzyme activities in C57BL/KsJ-db/db mice. Life Sci. 2006; 79: 1207–1213
- Kojima T, Uesugi T, Toda T, Miura Y, Yagasaki K. Hypolipidemic action of the soybean isoflavones genistein and genistin in glomerulonephritic rats. Lipids. 2002; 37(3)261–265
- Gutt M, Davis CL, Spitzer SB. Validation of the insulin sensitivity index (ISI0,120): Comparison with other measures. Diabetes Res Clin Pract. 2000; 47: 177–184
- Matthews DR, Hosker JP, Rudenski AS. Homoeostasis model assessment: insulin and beta-cell function from fasting plasma glucose and insulin concentration in man. Diabetologia. 1985; 28: 412–419
- Katz A, Nambi SS, Mather K, Baron AD, Follmann D, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000; 85: 2402–1240
- Niehaus WG, Samuelson B. Formation of malondialdehyde from phospholipids arachidonate during microsomal lipid peroxidation. Eur J Biochem. 1986; 6: 126–130
- Ellman GL. Tissue sulfphydryl groups. Arch Biochem Bio Phys. 1959; 82: 70–77
- Ali BH, Mousa HM. Effect of dimethylsulfoxide on gentamicin-induced nephrotoxicity in rats. Human Exp Toxicology. 2001; 20(4)199–203
- Fabris B, Candido R, Armini L, Fischetti F, Calci M, Bardelli M. Control of glomerular hyper filtration and renal hypertrophy by an angiotensin converting enzyme inhibitor prevents the progression of renal damage in hypertensive diabetic rats. J Hypertens. 1999; 17(12)1925–1931
- Yamada H, Hishida A, Kumagai H, Nishi S. Effects of age, renal diseases and diabetes mellitus on renal size reduction accompanied by the decrease on renal function. Nippon Jinzo Gakkai Shi. 1992; 34(10)1071–1075
- Bwititi P, Musabayane CT, Nhachi CFB. Effects of Opuntia megacantha on blood glucose and kidney function in streptozotocin diabetic rats. J Ethnopharmacology. 2000; 69: 247–252
- Sanchez-Lozada LG, Tapia E, Santamaria J, Avila-Casado C, Nepomuceno T, Rodriguez-Iturbe B, et al. Mild hyperurcemia induces severe cortical vasoconstriction and perpetuates glomerular hypertension in normal rats and in experimental chronic renal failure. Kidney Int. 2005; 67: 237–247
- Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, et al. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005; 67: 1739–1742
- Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001; 38: 1101–1106
- Kang D-H, Park SK, Lee IK, Johnson RJ. Uric acid induced C-reactive protein (CRP) expression: Implication on cell proliferation and nitric oxide production in human vascular cells. J Am Soc Nephrol. 2005; 67: 1739–1742
- Hallfrisch J. Metabolic effects of dietary fructose. FASEB J. 1990; 4: 2652–2660
- Cosenzi A, Berobich E, Bonavita M, Gris F, Odoni G, Bellini G. Role of nitric oxide in the early renal changes induced by high-fructose diet in rats. Kidney Blood Press Res. 2002; 25(6)363–369
- Nakagawa T, Tuttle KR, Short RA, Johnson RJ. Fructose- induced hyperuricemia as a causal mechanism for the epidemic of the metabolic syndrome. Nat Clin Pract Nephrol. 2005; 1: 80–86
- Hoffman RS, Martino JA, Wahl G, Arky RA. Fasting and refeeding. Antinatriuretic effect of oral and intravenous carbohydrates and its relationship to potassium excretion. Metabolism. 1971; 20: 1065–1073
- Pedraza-Chaverri J, Barrera D, Hernandez-Pando R. Soy protein diet ameliorates renal nitrotyrosine formation and chronic nephropathy induced by puromycin aminonucleoside. Life Sci. 2007; 74: 987–999
- Lee JS. Effects of soy protein and genistein blood glucose, antioxidant enzyme activities and lipid profile in streptozotocin-induced diabetic rats. Life Sci. 2006; 79(16)1578–1584
- Choi C, Cho H, Park J, Cho C, Song Y. Suppressive effects of genistein on oxidative stress and NFκB activation in RAW 264.7 macrophages. Biosci Biotechol Biochem. 2003; 67(9)1916–1922
- Wu H-J, Chan W-H. Genistein protects methylglyoxal-induced oxidative DNA damage and cell injury in human mononuclear cells. Toxicology in Vitro. 2007; 21: 335–342
- Mahn K, Borras C, Knock GA, Taylor P, Khan IY, Sugden D. Dietary soy isoflavone-induced increases in antioxidant and eNOS gene expression lead to improved endothelial function and reduced blood pressure in vivo. FASEB J. 2005; 10: 1–8
- Echiffrin EL. Enthelin: Role in experimental hypertension. Cardiovasc. Pharmacol. 2002; 35(4)533–535
- Sowers JR. Insulin resistance and hypertension. Am J Phyhsiol Heart Circ Physiol. 2004; 286: H1597–H1602
- Araki E, Murakami T, Shirotani T. A cluster of four SPI binding sites required for efficient expression of the human insulin receptor gene. J Biol Chemistry. 1991; 266(6)3944–3948