Abstract
Cisplatin is an effective chemotherapeutic agent used in the treatment of a wide array of both pediatric and adult malignancies. Dose-dependent and cumulative nephrotoxicity is the major toxicity of this compound, sometimes requiring a reduction in dose or discontinuation of treatment. Recent evidence has implicated oxidative and nitrosative stress in cisplatin-induced nephrotoxicity. Aphanizomenon flos-aquae (AFA), blue-green algae, is claimed to be a potential antioxidant. The present study was designed to explore the renoprotective potential of AFA against cisplatin-induced oxidative stress and renal dysfunction. The ethanolic extract of Aphanizomenon flos-aquae (EEAFA) (25, 50, 100 mg/kg−1 p.o.) was administered two days before through three days after cisplatin challenge (5 mg/kg−1 i.p.). Renal injury was assessed by measuring serum creatinine, blood urea nitrogen, creatinine and urea clearance, and serum nitrite levels. Renal oxidative stress was determined by renal TBARS levels, reduced glutathione levels, and enzymatic activity of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), and glutathione transferase (GST). A single dose of cisplatin produced marked renal oxidative and nitrosative stress and significantly deranged renal functions. Chronic EEAFA treatment significantly and dose-dependently restored renal functions, reduced lipid peroxidation, and enhanced reduced glutathione levels, superoxide dismutase, and catalase activities. The results of the present study clearly demonstrate the pivotal role of reactive oxygen species and their relation to renal dysfunction and point to the therapeutic potential of AFA in cisplatin-induced nephrotoxicity.
INTRODUCTION
Cisplatin [cis-diamminedichloroplatinum (II), CIS] is a highly effective antineoplastic DNA alkylating agent that is used to treat many types of solid tumors, including testicular, ovarian, breast, lung, bladder, and head and neck. However, reversible and irreversible side effects including nephrotoxicity, bone marrow toxicity, gastrointestinal toxicity, neurotoxicity, and ototoxicity may limit its utility and therapeutic profile.Citation[1] The free radicals play a pivotal role in the development of CIS-induced oxidative stress and renal dysfunction.Citation[2] CIS-induced acute renal failure is closely associated with enhanced lipid peroxidation.Citation[3] It has been reported that CIS enhances the generation of hydrogen peroxide and hydroxyl radicals, induces glutathione depletion, and increases peroxynitrite anion.Citation[4],Citation[5]
Recent studies have demonstrated that in certain micro algae, a blue protein called phycocyanin belonging to the photosynthetic apparatus has antioxidant and free radical scavenging properties in both in vivo and in vitro models.Citation[6] Most of the species belonging to the order Nostocales and Oscillatoriales are known to have renoprotective and biomodulator effects.Citation[7] A. flos-aquae (AFA) is a cyanobacteria belonging to the order Nostocales, and no work in this respect has been carried out with AFA until this report.
Focusing our attention on natural and bioavailable sources of antioxidants, we undertook to investigate the antioxidant and renoprotective effects of the cyanophytes AFA, a fresh water unicellular blue-green alga, is consumed as a nutrient-dense food source and for its health-enhancing properties. AFA is an important source of the blue photosynthetic pigment phycocyanin (PC), which has been described as a strong antioxidantCitation[8],Citation[9] and natural anti-inflammatoryCitation[10],Citation[1] compound, as evidenced by in vitro and in vivo studies on PC from the cyanophytes Spirulina platensis. PC is a water-soluble phycobiliprotein composed of h subunit polypeptides which associate into (ah)-monomers,Citation[12] which, in turn, have a high affinity to assemble together to form (ah)3-trimers and finally (ah)6-examers. a and h subunits are constituted of a protein backbone to which linear tetrapyrrole chromophoric groups are covalently bound.Citation[13] The chromophore, named phycocyanobilin is similar in chemical structure to bilirubin, and like the latter acts as a powerful scavenger of reactive oxygen species.Citation[14],Citation[15] The objective of the present work is to explore the renoprotective activity of AFA on CIS-induced oxidative stress and renal dysfunction in rats.
MATERIALS AND METHODS
Animals
Male albino rats of Sprague-Dawley strain weighing between 120–150 g were purchased from Small Animal Breeding Section of Kerala Agriculture University, Mannuthy, Trichur, Kerala, India. The animals were maintained in an animal house with standard facilities having CPCEA approval (No. 732). The animals were housed in polypropylene cages and maintained at 25 ± 2°C under 12 hour light/dark. They were fed with Amrut Laboratory Animal Feed, manufactured by Nav, Maharashtra Chakan Oil Mills, Ltd., Pune, India. Water was provided ad libitum. The animals were acclimatized for one week under laboratory conditions. Ethical clearance for handling the animals was obtained from the ethic committee constituted for the purpose.
Drugs
CIS (Sigma) was suspended in 0.25% carboxymethyl cellulose (CMC). Cyanobacteria strain, Aphanizomenon flos-aquae, procured from NCCUBGA, IARI New Delhi was used for the experiments.
Maintenance of Cyanobacteria Strain
Stock culture of the strains was maintained in our laboratory by using BG-11 medium.Citation[16] Cyanobacteria culture (50 mL) from a mid-log phase growth culture was dispersed aseptically into cotton plugged 1000 ml sterilized conical flasks. These were maintained at 25°C ± 2°C under 24 hours light in an illuminated chamber at 2.5 Klux. These cultures were thoroughly shaken 2–3 times daily to prevent mat formation.Citation[17] Aeration was provided by using an aerator.
Preparation of Extract
The cyanobacteria culture was grown up to the mid-log phase (10–14 days old culture) and then it was harvested by filter removing the medium through coarse filter paper.Citation[18] Then, the harvested cyanobacteria mass was washed twice with distilled water for completely removing the culture medium.Citation[19] Approximately 8–10 ml water was used for every 1 gm culture harvested. The harvested strains of AFA were dried at 45–50°C for 48 hr. The dried material was granules, which were macerated in liquid nitrogen in a pestle and mortar until a fine powder was obtained. The powdered material was mixed with petroleum ether and sonicated to break open the cell wall (25 gm powder was mixed with 125 ml of solvent).Citation[20] Then it was placed on the shaker platform for 24 hours for cold extraction. The filtrate was collected by centrifugation, and the material was obtained and repeatedly cold extracted with solvents of increasing polarity (chloroform, ethanol, methanol, and distilled water). The filtrate was evaporated by rotary evaporator at temperature 30–35°C, and the mass obtained was dissolved in distilled water and employed for further experiments. The cold extraction procedure was repeated two times. The extract (300 mg) was dissolved in 10 ml of water, and each rat was orally fed with 500 μl of this preparation. Thus, each rat received approximately 15 mg of the drug (i.e., 100 mg/kg body w.t). The average body weight of each rat was 120–150 g.
Experimental Design
Animals were divided into five groups, each group comprising six animals. Group 1 was kept as normal that received equivalent volume of sodium carboxymethylcellulose for five days. Group 2 animals received single dose of CIS 5 mg kg−1.Citation[21] Groups 3–5 animals received ethanolic extract of Aphanizomenon flos-aquae (EEAFA) 25, 50, and 100 mg kg−1, respectively, starting two days before and continually until 3 days after CIS. The animals were kept starved overnight on the sixth day of the CIS treatment. On the next day, the animals were sacrificed by decapitation, and the blood was collected by cutting the jugular vein.Citation[22] The kidney in each case were dissected out, blotted of blood, washed in saline, and stored in a freezer, and they were used for various biochemical estimations.
Post-Mitochondrial Supernatant Preparation
Kidneys were perfused with ice-cold saline (0.9% sodium chloride) and homogenized in chilled potassium chloride (1.17%) by using a homogenizer. The homogenate were centrifuged at 800 g for 5 min at 4°C to separate the nuclear debris. The supernatant so obtained was centrifuged at 10,500 g for 20 min at 4°C to get the post-mitochondrial supernatant, which was used to assay antioxidant enzyme activities.Citation[21]
Biochemical Estimations
A midline abdominal incision was performed, and both kidneys were isolated for enzymatic analysis and histopathological studies. Plasma samples were assayed for blood urea nitrogen (BUN), urea clearance, serum creatinine, and creatinine clearance by using standard diagnostic kits (Doctor's diagnostics, Kerala, India). Assay was completed based on the ability of the extracts to inhibit or scavenge the super oxide radical generated from the photo reduction of riboflavin according to the method of McCord and Fridovich.Citation[23] The measurement of thiobarbituric acid reactive substances (TBARS) was done as an index of lipid peroxidation by using the method of Nichans and Samuelson.Citation[24] Conjugated dienes (CD) was estimated according to the method of Beuje and Aust.Citation[25] Activities of superoxide dismutase (SOD),Citation[26] catalase (CAT),Citation[27] reduced glutathione (GSH),Citation[28] glutathione peroxidase (GPX),Citation[29] glutathione transferase (GST),Citation[30] and serum nitrite concentrationCitation[31] were also estimated.
Histopathological Examination of Kidney
A portion of the kidney in each group was fixed in 10% formasal (formalin diluted to 10% with saline) and protected for histopathology. For histopathology, serial sections of 5μm thickness were made from the fixed kidney tissues and then studied with hematoxylin and eosin to evaluate the details of renal architecture in each group microscopically. A minimum of 10 fields of kidney slides were examined and assigned for severity of changes by an observer blinded to the treatments of the animals and assigned for severity of changes by using scores on the scale of none (−), mild (+), moderate (++), and severe (+++).
Statistical Analysis
The results are presented as the mean of six animals in each group ± SD. The data were obtained by one-way ANOVA followed by Dunnett t-test.Citation[32] The level of significance was set at p < 0.01.
RESULTS
Effect of EEAFA on CIS-Induced Renal Dysfunction
The single administration of CIS significantly (p < 0.01) increased the serum creatinine and blood urea nitrogen (BUN). Chronic EEAFA treatment significantly and dose-dependently prevented the rise in BUN and serum creatinine (see ). Moreover, the creatinine and urea clearance, which were markedly reduced by CIS administration, was improved dose-dependently by EEAFA treatment.
Table 1 Effect of ethanol extract of Aphanizomenon flos-aquae on CIS-induced nephrotoxicity in rats
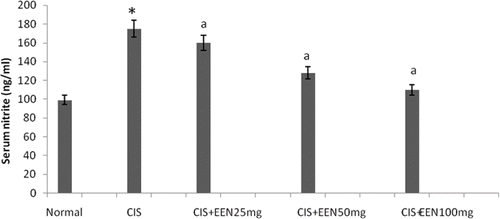
Effect of EEAFA on CIS-Induced Nitrosative Stress
Serum nitrite levels were significantly (p < 0.01) elevated by CIS administration. EEAFA treatment significantly and dose-dependently improved this increase in serum nitrite levels (see ).
Effect of EEAFA on CIS-Induced Lipid Peroxidation and Changes in Antioxidant Profile
A pilot study was carried out by using the extracts of AFA in different organic solvents. Among the extracts, extract with ethanol showed maximum antioxidant activity (see ), which was selected for the studies. TBARS and CD levels were increased significantly by CIS administration compared with normal control group of animals. Treatment with EEAFA produced a significant (p < 0.01) reduction in TBARS and CD in CIS-treated animals (see ). Antioxidant enzyme activities of kidney are presented in . SOD, CAT, GST, and GPX activities were reduced significantly (p < 0.01) in the CIS-intoxicated rats when compared with normal rats. In CIS+ EEAFA-treated rats, the activities of these enzymes attained near normal levels. The effect of EEAFA seems to be dose dependent. Conversely, GSH content in the kidney of group 2 animals showed a significant (p < 0.01) decline when compared with group 1. But in all other groups of animals, GSH content was found to attain near normal levels.
Figure 2. In vitro superoxide scavenging activity of ethanol, methanol, and aqueous extract of A. flos-aquae.
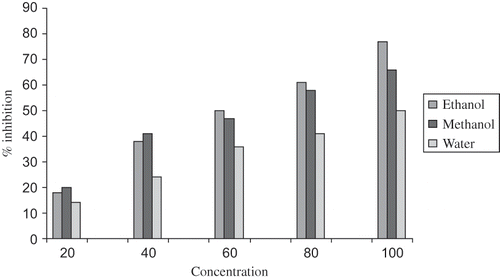
Table 2 Effect of ethanol extract of Aphanizomenon flos-aquae on lipid peroxidation in CIS-treated rats
Table 3 Effect of ethanol extract of Aulosira fertilisima on activity of antioxidant enzymes in kidney of CIS-treated animals
Effect of EEAFA on CIS-Induced Changes on Renal Morphology
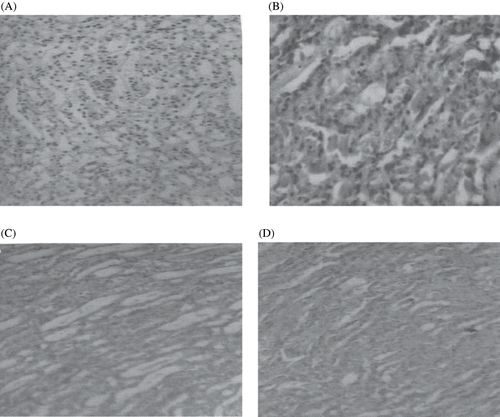
The histopathological changes were graded and summarized in . The kidneys of rats treated with CIS (see , plate 2) showed marked histological changes in the cortex and outer medulla, such as tubular brush border loss, interstitial edema, and necrosis of epithelium, as compared with normal renal (plate 1) histopathological structure. Treatment with EEAFA regressed most of these changes produced by CIS (plates 3 and 4).
Table 4 Effect of Aphanizomenon flos-aquae treatment on morphological changes as assessed by histopathological examination of kidney in CIS-treated rats
Figure 3. Photomicrographs of kidney sections of rat stained with hematoxylin and eosin (×100). (A) Haematoxylin- and eosin-stained sections of normal rat kidneys; (B) kidney section of CIS-treated rats showing tubular brush border loss and necrosis of epithelium; (C) kidney section of CIS + EEAFA, 50 mg-treated rats showing prevention of CIS-induced alterations; (D) kidney section of CIS + EEAFA, 100 mg-treated rats showing prevention of CIS-induced alterations.
DISCUSSION
Oxidative stress plays a key role in CIS-induced renal dysfunction.Citation[33] CIS has been reported to enhance production of superoxide,Citation[34] peroxynitrite,Citation[35] hydrogen peroxide,Citation[36] and hydroxyl radicals via the metabolization of iron from renal cortical mitochondria.Citation[37] Because almost all ATP is produced in mitochondria, mitochondrial dysfunction is a central component of CIS nephrotoxicity to proximal tubules in vivo and in vitro.Citation[38] Renal proximal tubule cells (PTCs), which accumulate significantly greater amounts of CIS in vivo and in vitro than other nephron segments, undertakes substance transport by consumption of large amounts of ATP. PTCs are, therefore, highly sensitive to any disturbance in ATP production.
Ample experimental and epidemiological studies support the involvement of oxidative stress in the pathogenesis and progression of several chronic diseases.Citation[39] It has been reported that CIS nephrotoxicity is associated with increased lipid peroxidation in renal cortical slices and that antioxidants reverse CIS-induced lipid peroxidation.Citation[40] In the present study, we found that EEAFA could effectively scavenge the free radicals in a dose-dependent manner. In the present study, elevated level of TBARS and CD observed in CIS-treated rats indicate excessive formation of free radicals and the activation of lipid peroxidation system resulting in kidney damage. The significant decline in the concentration of these constituents in the kidney of CIS+EEAFA-treated rats indicates anti–lipid peroxidative effect of AFA. The antioxidant property of Aulosira fertilisima and Nostoc sphaeroides on CCl4-induced hepatic damage in rats had been reported from earlier works conducted in our laboratory.Citation[41],Citation[42] It has been reported that Spirulina fusiformis effectively inhibited CIS-induced lipid peroxidation in rat kidney in vivo.Citation[21] GSH is a major non-protein thiol in living organisms that plays a central role in coordinating the body's antioxidant defense processes.Citation[43],Citation[44] A decline in the GSH content in the liver of CIS-intoxicated rats and its subsequent return toward the near normal levels in CIS+EEAFA-administered group also reveal an anti-lipid peroxidative effect of AFA against CIS-induced renal dysfunction.
The body has an effective mechanism to prevent and neutralize the free radical-induced damage. This is accomplished by a set of endogenous antioxidant enzymes, such as SOD, CAT, GPX, and GST. When the balance between ROS production and antioxidant defenses is lost, oxidative stress results, which, through a series of events, deregulates the cellular functions leading to various pathological conditions.Citation[44],Citation[45] Nevertheless, the expression of superoxide dismutase and glutathione peroxidase genes is down-regulated by CIS.Citation[46] Any compound, natural or synthetic, with antioxidant properties might contribute toward the partial or total alleviation of this type of damage. In the present study, a decline in the level of antioxidant enzymes like SOD, CAT, GPX, and GST observed in the CIS-treated rat is a clear manifestation of excessive formation of free radicals and activation of lipid peroxidation system resulting in tissue damage. The significant increase (p < 0.01) in the concentration of these constituents in the kidney of CIS+EEAFA-treated animals indicates the antioxidant effect of EEAFA. The antioxidant activity (in vitro) of Nostoc sphaeroides and Spirulina maxima has been reported.Citation[47],Citation[48] It was reported through animal models that Dunaliella salina, a green marine algae, has the ability to protect against oxidative stress in vivo.Citation[19] It has been established that carotenoids from micro algae exert their action against lipid peroxidation, either through decreased production of free radical derivatives or due to the antioxidant activity of the protective agent itself.Citation[49]
Superoxide dismutase (SOD), one of the important intracellular antioxidant enzymes present in all aerobic cells, has an antitoxic effect against superoxide anion.Citation[50] Catalase is a hemoprotein that protects cells from the accumulation of H2O2 by dismutating it to form H2O and O2 or by using it as an oxidant in which it works as a peroxidase.Citation[51] GST is another scavenging enzyme that binds to many different lipophilic compounds. It acts as an enzyme for GSH conjugation reaction. It catalyses the reaction of hydroperoxides with reduced glutathione to form glutathione disulphide (GSSG) and the reduction product of hydroperoxide.
Experimental evidence has suggested that CIS deteriorates renal functions in a dose-dependent manner.Citation[52] Glomerular filtration rate (GFR) of both kidneys represents the integration of GFR in all functioning nephrons. The cause of the decrease in glomerular filtration is afferent vasoconstriction and possibly an altered ultra filtration coefficient.Citation[53] At the single nephron level, ROS may decrease effective GFR by adversely affecting the determinants of single nephron glomerular filtration rate (SNGFR) and by predisposing to back leak of ultrafiltrate across the tubular epithelium. Thus, ROS may contribute to the reduction of GFR in acute tubular necrosis (ATN) by adversely affecting the determinants of glomerular hemodynamics, injuring tubular epithelial cells, and provoking tubular cast formation.Citation[54]
In the present study, the single administration of CIS caused marked renal dysfunction as evidenced by decreased creatinine and urea clearance. CIS predictably lowers GFR in a dose-dependent manner, the cause for which is afferent vasoconstriction and possibly an altered ultrafiltration coefficient.Citation[55] Results of the present study showed that EEAFA significantly improved creatinine and BUN. It may be possible that AFA, due to its potential antioxidant properties, improved renal functions via attenuating oxidative stress-mediated decline in GFR and renal hemodynamics. The vasodilating property of Aphanizomenon on rat aortic rings, possibly depending on a cyclo-oxygenase-dependent product of arachidonic acid and nitric oxide, has been reported.
Nitrite, the stable end product of nitric oxide metabolism, reacts with superoxide radicals, which finally leads to nitrosative stress. Large amounts of nitric oxide can lead to the depletion of cellular ATP, which can inactivate enzymes that contain iron-sulphur clusters, such as the TCA cycle enzyme aconitase, and enzymes involved in mitochondrial electron transport. Nitric oxide damages DNA, which in turn stimulates the DNA repair enzyme poly-ADP-ribose synthetase, which further exacerbates ATP depletion and reduces cellular levels of NAD.Citation[56] By depleting ATP and promoting ADP ribosylation, nitric oxide may impair cytoskeleton integrity. In the present study, CIS produced severe nitrosative stress as assessed by marked increase in serum nitrite levels, which was significantly and dose-dependently attenuated by EEAFA probably due to the inhibition of inducible nitric oxide synthase.
It has been reported that ROS may lead to tubular damage in CIS-treated rats. ROS can induce either sublethal or lethal cell injury that culminates in either necrosis or apoptosis. The kidneys of CIS-treated rats showed marked histological alterations, especially in the outer cortex and medullary region of the kidneys, compared with kidneys of normal control group. EEAFA significantly regressed these structural changes in the kidney, suggesting the possible involvement of ROS in mediating these histological alterations.
The present study provides convincing evidence for the oxidative stress-related renal dysfunction and morphological alterations in this rat model of CIS-induced ARF. It demonstrates the renoprotective and antioxidant properties of ethanol extract of A. flos-aquae. The renoprotective effect of EEAFA may be due to the presence of phycocyanin pigment present in EEAFA. In this regard, it is relevant to point out that phycocyanin has been suggested to act as an antioxidant and exert its antioxidant activity by scavenging lipid peroxidation. Thus, the plausible mechanism of the renoprotective effect of EEAFA may be due to its antioxidant effect. Further study is needed to identify and isolate the active principle of EEAFA, which can have offer antioxidant and renoprotective properties.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Arany I, Safirstein RL. Cisplatin nephrotoxicity. Semin Nephrol. 2003; 23: 460–464
- Rao NK, Nammi S. Antidiabetic and renoprotective effects of the chloroform extract of Terminalia chebula Retz. seeds in streptozotocin-induced diabetic rats. Complementary and Alternative Medicine. 2006; 6: 17–22
- Kotins MS, Patel P, Menon SN, Sane RT. Renoprotective effect of Hemidesmus indicus, a herbal drug used in gentamicin-induced renal toxicity. Nephrology. 2004; 9(3)142–147
- Zhang JG, Lindup WE. Cisplatin nephrotoxicity decreases the mitochondrial protein sulphydryl concentration and calcium uptake by mitochondria from rat renal cortical slices. Biochem Pharmacol. 1994; 47: 1127–1135
- Lind DS, Kontaridis MI, Edwards PD, Joseph MD, Copeland EM, III. Nitric oxide contributes to Adriamycin anti-tumor effect. J Surg Res. 1997; 69: 283–287
- Chu HJ, Tsang CT. Research and utilization of cyanobacteria in China: A report. Arch Hydrobiol Suppl. 1988; 80: 1–6
- Romay C, Armesto J, Remirez D, Gonzalez R, Ledon L, Garcia I. Antioxidant and anti-inflammatory properties of C-phycocyanin from blue-green algae. Inflamm Res. 1998; 47: 36–43
- Bhat VB, Madyastha KM. C-phycocyanin: A potent peroxyl radical scavenger in vivo and in vitro. Biochem Biophys Res Commun. 2000; 275(1)20–25
- Romay C, Gonzalez R. Phycocyanin is an antioxidant protector of human erythrocytes against lysis by peroxyl radicals. J Pharm Pharmacol. 2000; 52(4)367–368
- Bhat VB, Romay C, Armesto J, Remirez D, Gonzalez R, Ledon N, Garcia I. Antioxidant and anti-inflammatory properties of C-phycocyanin from blue-green algae. Inflammation Res. 1998; 47(1)36–41
- Reddy CM, Bhat VB, Kiranmai G, Reddy MN, Reddanna P, Madyastha KM. Selective inhibition of cyclooxygenase-2 by C-phycocyanin, a biliprotein from Spirulina platensis. Biochem Biophys Res Commun. 2000; 277(3)599–603
- Glazer AN, Stryer L. Phycobiliprotein-avidin and phycobiliprotein-biotin conjugates. Methods in Enzymology. 1990; 184: 188–194
- Padyana AK, Bhat VB, Madyastha KM, Rajashankar KR, Ramakumar S. Crystal structure of a light harvesting protein C-phycocyanin from Spirulina platensis. Biochem Biophys Res Commun. 2001; 282: 893–898
- Stocker R, McDonagh AF, Glazer AN, Ames BN. Antioxidant activities of bile pigments: Biliverdin and bilirubin. Methods in Enzymology 1990; 186: 301–309
- Bhat VB, Madyastha KM. Scavenging of peroxynitrite by phycocyanin and phycocyanobilin from Spirulina platensis: Protection against oxidative damage to DNA. Biochem Biophys Res Commun. 2001; 285(2)262–266
- Stainer RY, Kunisawa R, Mandel M, Cohen Bazire G. Purification and properties of unicellular cyanobacteria (Order: Chroococcales). Bacteriol Rev. 1971; 35: 171–176
- Rippka R, Deruelles J, Waterbury JB, Herdeman M, Stainer RY. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiology. 1979; 111: 1–7
- Uday B, Dipak D, Banerjee E, Ranjith K. Reactive oxygen species: Oxidative damage and pathogenesis. Curr Sci. 1999; 5: 658–664
- Chidambara Murthy KN, Rajesha J, Mahadeva Swamy M, Ravishankar GA. Comparative evaluation of hepatoprotective activity of carotenoids of microalgae. J Med Food. 2005; 8(4)523–527
- Ha K-T, Yoon S-J, Choi D-Y, Kim D-W, Hokim C. Protective effect of Lycium chinese fruit on carbon tetrachloride induced hepatotoxicity. J Ethanopharmacol. 2005; 96: 529–533
- Kuhad A, Tirkey N, Pilkhwal S, Chopra K. Renoprotective effect of Spirulina fusiformis on cisplatin-induced oxidative stress and renal dysfunction in rats. Ren Fail. 2006; 28(3)247–253
- Venukumar MR, Latha MS. Hepatoprotective effect of methanolic extract of Curculigo orchioides in CCl4 treated male rats. Indian J Exp Biol. 2004; 42: 792–798
- McCord JM, Fridovich I. Superoxide dismutase, an enzymatic function for erythrocuprein. J Biol. Chem. 1969; 224: 6049–6054
- Nichans WG, Samuelson B. Formation of MDA from phospholipids arachidonate during microsomal lipid peroxidation. Eur J Biochem. 1968; 6: 126–132
- Beuje JA, Aust SD. Methods in Enzymology, SP Clowick, NO Kaplan. Academic Press, New York 1978; 52: 302–306
- Kakkar P, Das B, Vishwanath DN. A modified spectro-photometric assay of superoxide dismutase. Indian J Biochem. Biophys. 1984; 21: 130–134
- Calibrone AL. Handbook of Methods for Oxygen Radical Research. CRC Press, Boca Raton, Fla 1985; 283–287
- Bentler E, Kelly BM. The effects of sodium nitrate on red cell glutathione. Experientia. 1963; 19: 96–97
- Rotruck JT, Pope AL, Gantter HE. Selenium: Biochemical roles as a component of glutathione peroxidase. Science. 1979; 588–593
- Beutler E. Red Cell Metabolism—a Manual of Biochemical Methods. Gruene, Stratton, New York 1986; 57–62
- Green LC, Wagner DA, Glogowski J, Skipper PL. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 1982; 126: 131–138
- Girish Achliya S, Sudhir Wadodkar G, Avinash Dorle K. Evaluation of hepatoprotective effect of Amalkadi Ghrita against carbon tetrachloride induced hepatic damage in rats. J Ethanopharmacol. 2004; 90: 229–335
- Baliga R, Ueda N, Walker PD, Shah SV. Oxidant mechanisms in toxic acute renal failure. Drug Metab Rev. 1999; 31: 971–997
- Matsushima H, Yonemura K, Ohishi K, Hishida A. The role of oxygen free radicals in cisplatin-induced acute renal failure in rats. J Lab Clin Med. 1998; 131: 518–526
- Davis CA, Nick HS, Agarwal A. Manganese superoxide dismutase attenuates CIS-induced renal injury: Importance of superoxide. J Am Soc Nephrol. 2001; 12: 2683–2690
- Srivastava RC, Farookh A, Ahmad N, Misra M, Hasan SK, Hussain MM. Evidence for the involvement of nitric oxide in CIS-induced toxicity in rats. Biometals. 1996; 9: 1, 39–142
- Rao M, Kumar MM, Rao MA. In vitro and in vivo effects of phenolic antioxidants against CIS-induced nephrotoxicity. J Biochem (Tokyo). 1999; 125: 383–390
- Saad SY, Najjar TA, Noreddin AM, Al-Rikabi AC. Effcets of gemcitabine on CIS-induced nephrotoxicity in rats: Schedule-dependent study. Pharmacol Res. 2001; 43: 193–198
- Lynch ED, Gu R, Pierce C, Kil J. Reduction of acute cisplatin ototoxicity and nephrotoxicity in rats by oral administration of allopurinol and ebselen. Hear Res. 2005; 201: 81–84
- Kuhad A, Pilkhwal S, Sharma S, Tirkey N, Chopra K. 6-gingerol prevents cisplatin-induced acute renal failure in rats. [My paper]Biofactors. 2006; 26(3)189–192
- Kuriakose GC, Thomson AM, Kurup MG. Antioxidant effect of Nostoc sphaeroides against carbon tetrachloride induced liver dysfunction in rats. Indian J Bot Res. 2006; 2(1)83–86
- Kuriakose GC, Kurup MG. Antioxidant activity of Aulosira fertilisima on CCl4 induced hepatotoxicity in rats. Ind J Exp Biol. 2008; 46: 52–56
- Campos R, Garrido A, Valenzuela A, Guerra R. Silybin dihemisuccinate protects against glutathione depletion and lipid peroxidation induced by acetaminophen on rat liver. Planta Med. 1989; 55: 417–421
- Valenzuela A, Lagos C, Schmidt K, Videla K. Silymarin protection against hepatic lipid peroxidation induced by acute ethanol intoxication in the rat. Biochem Pharmacol. 1985; 3: 2209–2213
- Castro JA, Ferrya GC, Castro CR, Sasamett Fenos OM, Gillette JR. Prevention of carbon tetrachloride-induced necrosis by inhibitors of drug metabolism. Further studies on the mechanism of their action. Biochem Pharmacol. 1974; 23: 295–301
- Ferrari R, Ceconi C, Curello S, Cargnoni A, Paini E, Visloli O. The occurrence of oxidative stress during reperfusion in experimental animals and men. Cardiovasc Ther. 1991; 52: 77–82
- Kuriakose GC, Kurup MG. Antioxidant activity (in vitro) of N. sphaeroides. Indian J Bot Res. 2008; 4(1)54–59
- Miranda MS, Cintra RG, Barros SM, Mancini-Filho J. Antioxidant activity of the microalga Spirulina maxima. Braz J Med Biol Res. 1998; 31: 1075–1081
- Kerfeld C. Structure and function of water soluble carotenoid-binding protein of cyanobacteria. Photosyn Res. 2004; 81(3)215–219
- Rajmohan T, Loperito Loki A. Hepatoprotective and antioxidant effect of tender coconut water on carbon tetrachloride induced liver injury in rats. Indian J Biochem Biophys. 2003; 40: 354–358
- Bhakta T, Pulok M, Kakali K. Evaluation hepatoprotective activity of Cassia fistula leaf extract. J Ethanopharmacol. 1999; 66: 227–232
- Nagai J, Takano M. Molecular aspects of renal handling of amino glycosides and strategies for preventing the nephrotoxicity. Drug Metab Pharmacokinet. 2004; 19: 159–163
- Taguchi T. Cisplatin-associated nephrotoxicity and pathological events. Contrib Nephrol. 2005; 148: 107–111
- Rossert J. Drug-induced acute interstitial nephritis. Kidney Int. 2001; 60: 804–809
- Daugaard G, Abildggaad U. Cisplatin nephrotoxicity: A review. Cancer Chemother Pharmacol. 1989; 25: 1–6
- Rao M, Rao MN. Protective effects of cystone, a polyherbal ayurvedic preparation, on CIS-induced renal toxicity in rats. J Ethanopharmacol. 1998; 62: 1–6