Abstract
Vesicoureteral reflux (VUR) is a common congenital anomaly of the urinary tract that may be inherited. Reflux of infected urine may cause scarring in susceptible kidneys with the potential to compromise renal function. The aim of the study was to evaluate the possible influence of different grades of VUR on glomerular damage using microalbuminuria as a parameter. Children with VUR detected by voiding cystourethrography (VCUG) were investigated. According to the grade of VUR, patients were separated into three groups. The first group included 12 children with VUR grade I–II. The second group consisted of 12 children with grade III of VUR. Patients with VUR grade IV–V (n = 11) were members of the third group. The control group consisted of 17 healthy children. Microalbuminuria was examined in samples of morning urine specimens using a microalbumin/creatinine reagent kit. Serum urea, creatinine levels and creatinine clearance (CCR) were measured as markers of renal function. The mean value of microalbumin excretion in the third group showed a statistically significant increase (p < 0.001) compared to all other groups. CCR in the third group was statistically significantly decreased (p < 0.05) in comparison to the group of healthy children. There were no statistically significant changes of microalbumin excretion and CCR in the first and second group compared to control values. We discussed the presence of microalbuminuria and decrease of CCR in children with high grade of VUR as a possible consequence of retrograde urine flow (intrarenal reflux), glomerulosclerosis, and consecutive hyperfiltration.
Keywords:
INTRODUCTION
Primary vesicoureteral reflux (VUR) is a common congenital anomaly of the urinary tract that may be inherited. In early life, the most frequent clinical presentation is manifested as a complicating urinary tract infection (UTI). Dysfunction of ureterovesical junction causes reflux of infected urine from the bladder to ureters and pyelocalices (intrarenal reflux), which may cause scarring in susceptible kidneys and has the potential to compromise renal function.
The term reflux nephropathy (RN) has replaced chronic non-obstructive atrophic pyelonephritis to describe the small, contracted, irregularly scarred kidney, which clinically may present with UTIs, hypertension, proteinuria, or renal failure.Citation[1] The International Reflux Grading system classifies VUR into five grades, depending on the degree of retrograde filling and dilation of the renal collecting system.Citation[2] This system is based on the radiographic appearance of the renal pelvis and calyces on a voiding cystogram, as follows:
grade I: urine backs up into the ureter only, and the renal pelvis appears healthy, with sharp calyces.
grade II: urine backs up into the ureter, renal pelvis, and calyces. The renal pelvis appears healthy and has sharp calyces.
grade III: urine backs up into the ureter and collecting system. The ureter and pelvis appear mildly dilated, and the calyces are mildly blunted.
grade IV: urine backs up into the ureter and collecting system. The ureter and pelvis appear moderately dilated, and the calyces are moderately blunted.
grade V: urine backs up into the ureter and collecting system. The pelvis severely dilates, the ureter appears tortuous, and the calyces are severely blunted.
Microalbuminuria is unlikely if the microalbumin excretion rate is less than 20 μg/min in a timed urine collection or if the urine microalbumin concentration is less than 20–30 mg/L in a random specimen. The compounding effect of the urine volume can be avoided entirely by the calculation of the albumin-to-creatinine ratio (UMA/UCr) in the first morning urine specimen. A value above 30 mg/g suggests that microalbumin excretion is above 30 mg/day and therefore that microalbuminuria is probably present.Citation[3] Over the last two decades, a shift is paradigm has taken place regarding the role of proteinuria in the progression of renal disease. The degree of proteinuria has been shown to correlate with the risk of progression to end-stage renal disease. Initially, proteinuria was thought simply to be a marker of the severity of glomerular injury. Recent evidence, however, suggests that proteinuria, and particularly albuminuria, may itself promote tubular injury, with interstitial inflammation and eventual fibrosis that may be due to renal failure.Citation[4] Although the disease is well described in young children, there are only a few studies examining glomerular damage and microalbuminuria in children with VUR.
The aim of the study was to evaluate glomerular damage in children with different grades of VUR. Therefore, we measured microalbuminuria as a marker of glomerular damage. Serum urea, creatinine, and creatinine clearance (CCR) were examined as parameters of renal function.
PATIENTS AND METHODS
Children with VUR (n = 35, aged 1 month–16 years) detected by voiding cystourethrography (VCUG) were investigated. According to the grade of VUR, patients were separated into three groups: the first group included 12 children with VUR grade I–II, the second group consisted of 12 children with grade III of VUR, and the third group consisted of patients with VUR grade IV–V (n = 11). The control group consisted of 17 healthy children (aged 1–16 years).
Urine and blood samples were taken in the morning. Urinary microalbumin excretion was examined in samples of morning urine specimens using DCA 2000 microalbumin/creatinine reagent kit (Bayer Corporation, Elkhart, Indiana, USA) and expressed as microalbumin/creatinine ratio (mg/g). A value greater than 30 and less than 300 mg/g was considered as microalbuminuria. Serum urea and creatinine levels were measured using standard biochemical analysis. CCR was determined by the Schwartz Estimate: where Ht = height, Cr = serum creatinine level, and K = 0.45 for infants <1 year, 0.55 for children from 2–13 years and adolescent girls, and 0.70 for adolescent boys.
Results were expressed as the mean ± SD. The difference between means was verified by using analysis of variance (one-way ANOVA). Values of p < 0.05 were taken as statistically significant.
RESULTS
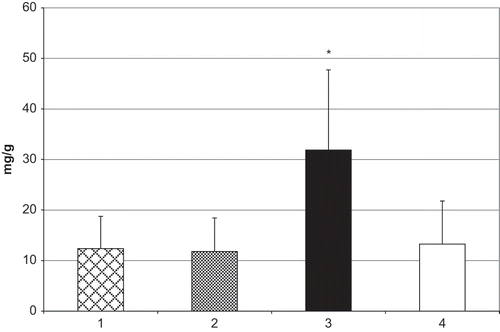
The mean value of microalbumin excretion in the third group (31.86±15.86 mg/g) showed a statistically significant increase (p < 0.001) compared to the first and second group and control values (see ).
Figure 1. Microalbumin/creatinine ratio in children with VUR. Group 1 = VUR grade I–II; group 2 = VUR grade III; group 3 = VUR grade IV–V; group 4 = control. *p < 0.001 vs. groups 1–3 and control.
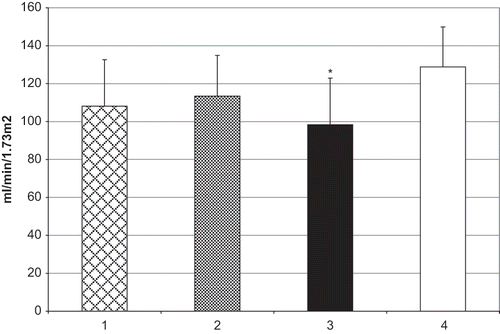
CCR in the third group was 98.37 ± 24.56 mL/min/1.73m2, which was a statistically significant decrease (p < 0.05) in comparison to the group of healthy children (128.81 ± 21.12; see ). There were no statistically significant changes of microalbumin excretion, or of CCR in the first and second groups compared to control values.
Figure 2. CCR in children with VUR. Group 1 = VUR grade I–II, group 2 = VUR grade III; group 3 = VUR grade IV–V; group 4 = control. *p < 0.05 vs. control.
There were no statistically significant changes of serum urea and creatinine levels in experimental groups compared to control (see ).
Table 1 Serum urea and creatinine levels
DISCUSSION
Primary vesicoureteral reflux, the retrograde flow of urine from the bladder into ureters and kidneys, affects 0.5–1% of the population and is, therefore, one of the more common congenital abnormalities. Infants or young children with VUR are highly susceptible to urinary tract infection.Citation[5],Citation[6]
In the majority of proteinuric states, albumin comprises the major protein fraction found in the urine. Epithelial cells lining the proximal tubule reabsorb filtered albumin through receptor-mediated endocytosis (e.g., via megalin).Citation[7] Albuminuria results when the filtered load of albumin exceeds the tubular reabsorptive capacity. Thus, urinary albumin represents an underestimate of the actual quantity of albumin to which tubular epithelial cells are exposed. Even in normal states, in which the glomerular filtration coefficient for albumin is quite low, the daily filtered load of albumin reaches an average of 1–2 g, virtually none of which appears in the urine.Citation[8] Thus, the tubular epithelial cells of even healthy individuals reabsorb a significant quantity of albumin. This raises the possibility that albumin may play a role in normal tubular homeostasis.Citation[4]
In this study, the mean value of microalbumin excretion (31.86±15.86 mg/g) in the group of children with high grade of VUR showed a statistically significant increase compared to all other groups. This value indicates the presence of microalbuminuria in the third group only.
Clinical and experimental studies pay more attention to different mechanisms of glomerular damage in VUR and RN. A high grade of vesicoureteral reflux causes retrograde urine flow from the bladder to ureters and pyelocalices due to RN. Intrarenal reflux or retrograde movement of urine from the renal pelvis into the renal parenchyma is a function of intrarenal papillary anatomy. Simple papillae possess oblique, slitlike, ductal orifices that close with increases in intrarenal pressure. Thus, simple papillae do not allow intrarenal reflux. However, compound papillae possess gaping orifices that are perpendicular to the papillary surface that remain open with increases in intrarenal pressure. These gaping orifices allow free intrarenal reflux. Two types of urine may enter the renal papillae: infected urine or sterile urine. Intrarenal reflux of infected urine appears to be primarily responsible for the renal damage. The presence of bacterial endotoxins (lipopolysaccharides) activates the host's immune response and a release of reactive oxygen species (ROS). The release of ROS and proteolytic enzymes results in fibrosis and scarring of the affected renal parenchyma and due to RN.Citation[2],Citation[9] On the other hand, a high grade of VUR causes retrograde urine flow from the bladder to ureters and pyelocalices, due to the inability of glomerular endothelium to release an adequate amount of endothelium-dependent vasodilatators (which induces a constriction at the efferent arteriole). Proximal to the constriction site at the efferent arteriole, it induces an elevation of intraglomerular hydrostatic pressure and hypertension. This leads to the reduction of the peritubular capillary flow, which can induce endothelial cell dysfunction by expressing adhesion molecules, proinflammatory cytokines, growth factors, ROS, and vasoconstrictors, namely angiotensin II with consecutive inflammatory process in the tubulointerstitium and RN.Citation[10]
When fibrosis during RN development causes the loss of 75% of functioning nephrons, glomerular hyperfiltration will be a compensatory mechanism in residual nephrons. This leads to higher permeability of the glomerular basal membrane (GBM) for proteins and consecutive tubular damage.Citation[9] Mesangial cells become activated and secrete cytokines and growth factors with consecutive focal-segmental glomerulosclerosis (FSGS). Secondary FSGS with non-nephrotic range proteinuria and without hypoalbuminemia or severe edema has been reported in patients with massive obesity, vesicoureteral reflux, or renal mass reduction.Citation[11–13] Therefore, the presence of microalbuminuria and decrease of CCR in children with a high grade of VUR in this study might be a possible consequence of retrograde urine flow (intrarenal reflux), glomerulosclerosis, and consecutive hyperfiltration. Also, microalbuminuria in children with a high grade of VUR might be a predictive parameter for high permeability of GBM and disease progression before proteinuria becomes manifest.
Although there were no changes in serum urea and creatinine levels, CCR in children with high grade of VUR showed a decrease in comparison to the group of healthy children. CCR is a better marker for the compromise of renal function than serum creatinine.Citation[14] Although albuminuria may itself promote tubular injury, with interstitial inflammation and fibrosis (RN) possibly occurring due to renal failure, decreased CCR in our study might be a sign of disease progression, and shows us a “signal” that RN is going to become clinically manifest soon.
Microalbumin excretion as well as CCR in the first (low grade of VUR) and second group (moderate grade) did not change compared to control values, which might indicate that there were fewer chances for glomerular damage and compromise of renal function in those groups of children.
Considering altered intrarenal hemodynamics, there are several studies suggesting the benefit of ACE inhibitors in patients with microalbuminuria and their protective effect on the kidney by preventing glomerular hypertrophy and, subsequently, glomerulosclerosis.Citation[15–17] However, only a few of them examined the role of this medication in children with VUR. Recently it has been suggested that there is a benefit of using ACE inhibitors.Citation[18]
In conclusion, the result of the present study suggests that the presence of microalbuminuria and decrease of CCR in children with a high grade of VUR are possible consequences of retrograde urine flow (intrarenal reflux), glomerulosclerosis, and consecutive hyperfiltration. The eventual use of urinary microalbumin measurement as a routine diagnostic test for high permeability of GBM in children with VUR as well as for the prevention of RN development when microalbuminuria is detected requires further investigation.
REFERENCES
- Bailey RR. Vesico-ureteric reflux and reflux nephropathy. Kidney Int. 1993; 42(44)S80–S85
- Popovic-Rolovic M, Peco-Antic A, Marsenic O. Vesicoureteral reflux and reflux nephropathy. Pediatric nephrology, M Popovic-Rolovic, A Peco-Antic, O Marsenic. Nauka, Belgrade, Serbia 2001; 68–77
- Assadi F. Quantification of microalbuminuria using random urine samples. Pediatr Nephrol. 2002; 17: 107–110
- Iglesias J, Levine JS. Albuminuria and renal injury—beware of proteins bearing gifts. Nephrol Dial Transplant. 2001; 16: 215–218
- Ping H, Fang-Ming D, Feng-Xia L, et al. Ablation of uroplakin III gene results in small urothelial plaques, urothelial leakage, and vesicoureteral reflux. J Cell Biology. 2000; 151(5)961–972
- Barroso JR, Barroso D, Jacobino M, Vinhaes A, Macedo A, Srougi M. Etiology of urinary tract infection in scholar children. Int Braz J Urol. 2003; 29: 450–454
- Brunskill NJ. Molecular interactions between albumin and proximal tubular cells. Exp Nephrol. 1998; 6: 491–495
- Gekle M. Renal proximal tubular albumin reabsorption: Daily prevention of albuminuria. News Physiol Sci. 1998; 13: 5–11
- Djukanovic LJ. Tubular function examining. Kidney disease, LJ Djukanovic, V Ostric. ZZUNS, Belgrade, Serbia 1999; 24
- Futrakul N, Laohaphaibul A, Futrakul P. Glomerular endothelial dysfunction and hemodynamic maladjustment in vesicoureteric reflux. Ren Fail. 2003; 25(3)479–483
- Balogun R, Adams N, Palmisano J, Yamase H, Chughtai I, Kaplan A. Focal segmental glomerulosclerosis, proteinuria and nephrocalcinosis associated with renal tubular acidosis. Nephrol Dial Transplant. 2002; 17: 308–310
- Praga M, Morales E, Herrero JC, et al. Absence of hypoalbuminemia despite massive proteinuria in focal segmental glomerulosclerosis secondary to hyperfiltration. Am J Kidney Dis. 1999; 33: 52–58
- Praga M, Borstein B, Andres A, et al. Nephrotic proteinuria without hypoalbuminemia: Clinical characteristics and response to angiotensin-converting enzyme inhibition. Am J Kidney Dis. 1991; 17: 330–338
- Leveratto IR, Torales MR, Clerc IM. True endogenous creatinine clearance value determined for the control of renal disease in children. Pediatria. 1996; 64(5)191–194
- Kliem V, Brunkhorst R, Ehlerding G, Kuhn K, Neumann KH, Koch KM. Prevention of glomerular hypertrophy and glomerulosclerosis in Milan normotensive rats by low-protein diet but not by low-dose captopril treatment. Nephron. 1995; 71: 208–212
- Kincaid-Smith P, Fairley K, Packham D. Randomized controlled crossover study of the effect on proteinuria and blood pressure of adding an angiotensin II receptor antagonist to an angiotensin converting enzyme inhibitor in normotensive patients with chronic renal disease and proteinuria. Nephrol Dial Transplant. 2002; 17: 597–601
- Harmankaya O, Seber S, Yilmaz M. Combination of pentoxifylline with angiotensin converting enzyme inhibitors produces an additional reduction in microalbuminuria in hypertensive type 2 diabetic patients. Ren Fail. May, 2003; 25(3)465–470
- Mattoo TK. Medical management of vesicoureteral reflux. Pediatr Nephrol. Aug, 2007; 22(8)1113–1120