Abstract
Introduction. In order to monitor acute renal failure, intensive care patients were examined, and routine as well as specialized parameters were compared. Materials and Methods. Thirty-three patients at the Surgical Intensive Care Unit (ICU) were examined daily over the entire period for which they stayed in the ICU. The patients were retrospectively classified as being either with or without acute renal failure. Group 1 consisted of 22 patients who resided in the ICU for 11–15 (median 14) days without ARF. Group 2 consisted of 11 patients who developed an ARF during their stay of 13–18 (median 16) days in the ICU. In addition to the routine parameters of diuresis, serum creatinine/urea, and clearance of creatinine, specialized parameters for kidney function, including the excretion rates of α1-microglobulin, N-acetyl-β-D-glucosaminidase, and total protein, were compared with the excretion rate of soluble ICAM-1 and sE-Selectin. Results. Diuresis, serum creatinine, urea, and enzyme elimination were pathological among patients with ARF. Already on the day of admission, raised elimination rates of sICAM-1 were found in the urine of patients who had developed an ARF. While high values were still shown upon discharge, levels kept falling among patients without ARF. Clearly raised values were also shown for sE-Selectin compared to patients without ARF. Conclusions. sICAM-1 and sE-Selectin as supplementary parameters indicating renal function revealed early signs of kidney damage. These parameters may play a major role in the development of novel therapeutic approaches for ARF (antibodies against ICAM-1 or sE-Selectin).
INTRODUCTION
One of the problems that patients can develop after severe traumas is an increased incidence of organ failure in the lungs, liver, and kidney; such events can be induced by hypoxia, hemodynamic alterations, or infection. Acute renal failure (ARF) still remains a frequent complication that entails severe consequences for intensive care patients. While solitary renal failure is treatable and the anuric period can be overcome comparatively well, the mortality of ARF is raised when multiple organ failure occurs.Citation[1] Restricted renal function is identified primarily by measuring creatinine clearance, dieresis, and the serum concentrations of creatinine and urea. These parameters, however, only become pathological during the later course of a kidney disease, at a time when the noxious agent (poisons, hypoxia, microembolism) is no longer necessarily active.
The spectrum of parameters examined included diuresis and the serum concentrations of creatinine and urea. α1-microglobulin (α1-MG) was measured to reveal structural alterations in tubular regions and as a specific parameter for tubular function.Citation[2],Citation[3] α1-MG is one of the best markers for disturbed tubular function. Increasing excretion rates are often seen before reductions in serum creatinine and generalized proteinuria are observed.Citation[3] N-acetyl-β-D-glucosaminidase (NAG) is a specific lysosomal marker present in large amounts in renal tubules. Increased excretion rates are seen in patients treated with specific tubulotoxic agents such as aminoglycosides.Citation[4] Total protein (TP) was recorded as a specific marker for glomerular function.Citation[5],Citation[6]
A number of studiesCitation[1],Citation[7–9] have addressed the special role of adhesion molecules in acute ischemia reperfusion kidney failure. ICAM-1 together with sE-Selectin plays a key role in the initiation and control of the apoptotic processes that occur during the development of acute kidney failure.Citation[10] The observations suggest a control function of sICAM-1 for preventing uncontrolled apoptosis and the irreversible destruction that occurs along with it.Citation[11]
sE-Selectin is expressed temporarily as a cell surface glycoprotein on endothelial cells after stimulation by cytokines.Citation[12] Because sE-Selectin in the area of the kidney epithelium is expressed only upon damage, it is conceivable that a raised sE-Selectin level in urine may act as an early indicator for kidney damage.Citation[13]
The introduction of the sICAM-1 and sE-Selectin as parameters for monitoring renal function has failed until today because their serum levels only poorly correlate with the pathological changes found in histological preparations.Citation[14] The question arises as to whether measurement of sICAM-1 and sE-Selectin represents a reasonable way to monitor acute kidney failure in afflicted or at-risk patients.
MATERIALS AND METHODS
In total, 33 patients from the Surgical Intensive Care Unit, Department of Anesthesiology, Justus-Liebig-University, Giessen, Germany, were examined after approval by the local ethical committee. The patients were divided retrospectively into two groups. Both groups were comparable regarding the conditions leading to admission to the intensive care unit and the ICU medical care treatment. The medication and ventilation therapy were also documented, and the APACHE-II-score was measured.
No patient showed clinical or laboratory signs of any renal functional insufficiency upon admission to the intensive care unit. Furthermore, any history of renal damage was excluded by employing contrast media.
Acute kidney failure is defined as a reduction in creatinine clearance to values below 40 mL/min and/or as an increase in serum creatinine to levels of at least 1.3 mg/dL/24h with unchanging levels of urine (polyuric/normouric kidney failure); those patients who develop an acute kidney failure are continuously hemofiltrated from the day of clinical onset.Citation[15]
For evaluating the examined parameters in urine and serum, values were recorded and compared on the day after admission, on day 7, until the day before discharge.
The spectrum of parameters examined included diuresis and the serum concentrations of creatinine and urea. α1-microglobulin (α1-MG) was employed as a specific parameter for tubular function, while N-acetyl-β-D-glucosaminidase (NAG) served as a specific marker for lysosomal structures. Total protein (TP) was recorded as a specific marker for glomerular function.
The plasma and urine samples were withdrawn from arterial or indwelling urine catheters, respectively. The withdrawn urine samples were centrifuged for 10 minutes at 3000 rpm in a Hettich Rotixa/KS centrifuge.
Serum Creatinine and Urea
Serum concentrations of creatinine were measured using an autoanalyzer (Model 30 R, WAKO Chemicals, Neuss, Germany). Measurement of urea in the serum samples was performed using a kinetic ultraviolet assay.
α1-Microglobulin (α1-MG) and TP
Urinary concentrations of α1-MG and TP were assessed by immunonephelometry (Behring nephelometer analyzer, Marburg, Germany).Citation[16] The proteins to be determined form immune complexes with their corresponding antisera. The amount of light scattered by an immune complex from a primary beam of light determines the concentration of the immune complex.
N-Acetyl- β -D-Glucosaminidase (NAG)
3-cresolsulfonephthaleinyl-N-Acetyl-ß-D-glucosaminide is hydrolytically cleaved by N-acetyl-β-D-glucosaminidase (NAG) so that 3-cresolsulfonephthalein is released, which is then measured photometrically at 580 nm. Addition of sodium carbonate arrests the reaction.Citation[17]
Intercellular Adhesion Molecule-1 (sICAM-1) and sE-Selectin in Serum and Urine
sICAM-1 and sE-Selectin concentrations were measured using an ELISA technique. No standardized reference values exist for urine.
Statistics
Descriptive statistics were first obtained for all the parameters. Mean values and standard errors of means (SEM) were determined. All parameters were tested for the normality of their distribution using the Fisher test.
A comparison of the parameters on the various examination days between patient groups with acute kidney failure and the patient group without acute kidney failure occurred for sE-Selectin in the urine, the only non-normally distributed parameter, using a modified form of the t-test for independent, non-normally distributed samples, with all other parameters being tested using the t-test for independent, normally distributed samples.
All tests were done in a two-tailed manner. The significance level was set to p < 0.05.
RESULTS
Patients, Scores, and Treatment Regimes
The patterns of injury amongst patients in both groups were comparable (see ). Regarding the biometric data, the hemodynamic parameters MAP, CVP, and APACHE-II scores were also comparable upon admission to the intensive care unit (see ).
Table 1 Patients‘ biometric data, hemodynamics, score values, and operation and transfusion data
Urea, Diuresis, Creatinine, and Creatinine Clearance
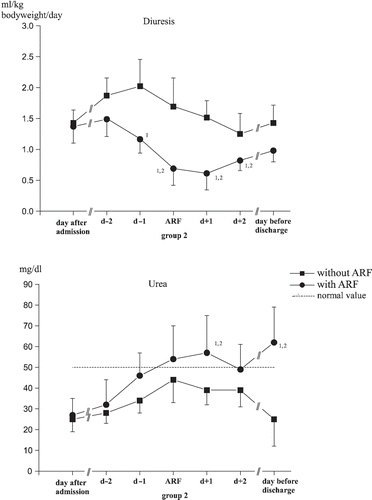
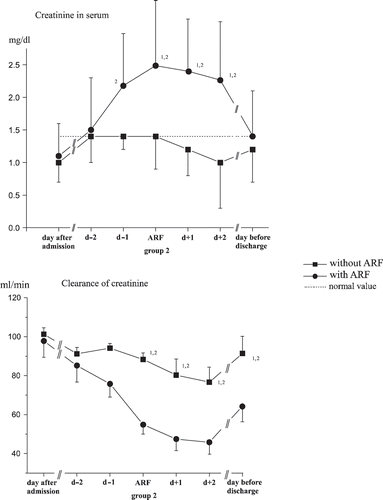
Normal diuretic rates were shown upon admission for all patients, and they decreased between the seventh and eighth days in the ICU for both groups (see ). Urea and plasma creatinine were within the normal range upon admission for all patients: Maximum levels were achieved between the fourth and eighth day of care among those who had suffered an acute renal failure (see and ). Creatinine clearance differed significantly between the groups at the point in time of acute renal failure until patient discharge (see ).
α1 Microglobulin, N-Acetyl-ß-D-Glucosaminidase, and Total Protein
For α1-MG, the elimination rates clearly increased in the group with acute kidney failure compared to initial values as well as the control group. Even at discharge, the values recorded upon admission were never reached again (see ).
Figure 3. Changes in excretion of α1-microglobulin (α1-MG) and N-Acetyl-ß-D-Glucosaminidase (NAG). (1significant differences to the time of admission, 2significant differences between the groups, p < 0.05.)
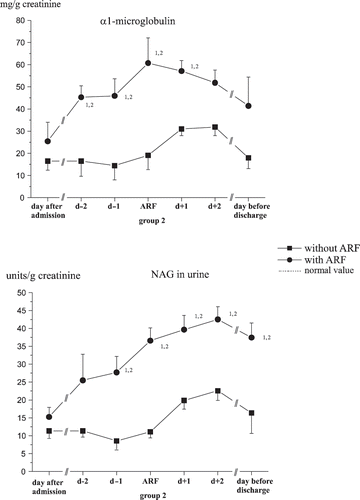
For 80% of all patients examined, there were increased elimination rates of the tubular marker α1-MG and NAG upon admission (see ). All examined patients showed raised excretion rates at some point during the intensive medical care. The maximum elimination rate was seen between the fifth and seventh days of care in each of the groups.
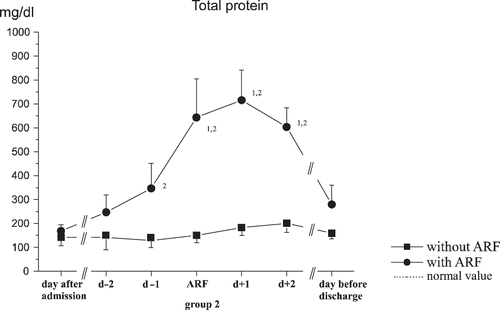
With total protein, the same initial values were shown, but there was a significant increase shown during acute kidney failure in group 2 (see ).
sICAM-1 in Urine
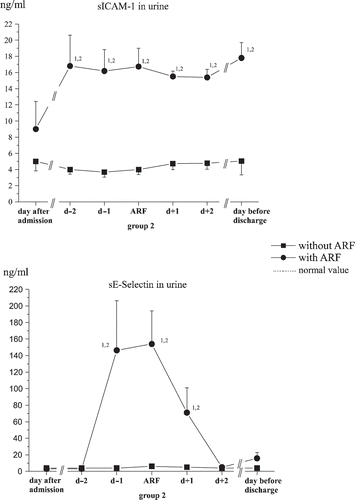
Within a few days of admission, significantly higher levels of sICAM-1 were found in the urine of ARF patients compared to the control collective. On the day before discharge, even higher values were found in the ARF group, while in the control group the levels remained the same (see ).
sE-Selectin in Urine
On the day after admission, no significantly different values were apparent for soluble sE-Selectin in urine between the two patient collectives.
On day 7, the mean values were raised significantly in the group that developed acute kidney failure, and on the day before discharge, the values were still higher than they were in the group that did not develop acute kidney failure (see ).
DISCUSSION
Acute renal failure is a potentially fatal complication among intensive care patients.Citation[18] The severity of the illness was illustrated by the APACHE-II scores (see ). This score was developed as a means to evaluate the severity of disease and in this way represents a satisfactory parameter for comparing the groups.
Diuresis, urea, and creatinine concentrations in serum as well as creatinine clearance behaved significantly differently in the two study groups, as indeed was expected (see and ).
Plasma urea concentrations varied significantly over time and differed significantly between the two groups from the sixth and seventh days of treatment. Here, it was not just the change in kidney function, but also the metabolic state and the activation of the renin-angiotensin-aldosterone system that played the major roles in determining the post-aggression phase (systemic inflammatory response syndrome) involving the increased accumulation of urea.Citation[19]
The sharp increase in the excretion rates of α1-MG and NAG already at the start of ICU treatment betrayed the early appearance of tubular damage. Such results confirm the early increases in urinary levels of this parameter described elsewhere in the literature.Citation[20],Citation[21] Here, the trauma itself plays a major role with fluid losses and hypotonic phases. The raised excretion rates following from the fifth to seventh days of treatment must have been due to intensive care measures such as controlled ventilation with PEEP, fluid substitution, or the administration of blood derivatives, colloids, or potentially toxic medications.
While serum creatinine and creatinine clearance only reach pathological levels when there is an organ loss amounting to at least 50% of the GFR, little can be said regarding the appearance of individual pathological protein and enzyme fractions in the urine of intensive care patients. Numerous investigations have confirmed the informative value of enzymes and proteins for specific situations such as kidney transplant rejection, drug overdosing, or intoxication.Citation[22] However, only a few reports have appeared regarding the etiology of renal dysfunction in surgical intensive care patients and the frequency distribution of its possible causes.Citation[23]
According to the literature, 80% of all cases of acute renal failure are caused by circulation-induced forms. However, a fully developed shock syndrome does not precede every case of renal failure. Vasoconstriction due to central involvement can already lead to ischemic kidney damage, while fully blown shock can actually be prevented by this. Here, the postoperative and posttraumatic statuses are worthy of special mention. The patients we examined were probably afflicted by circulation-induced renal dysfunction because the complete spectrum of renal structural damage was simultaneously found. Thus, an increase in tubular markers in urine (α1-MG, NAG) was demonstrated before a rise in plasma concentrations of creatinine and urea.
In 1997, Gauer et al. were able to verify that ICAM-1 is expressed in the area of the kidneys only if kidney damage occurs.Citation[24]
However, serum levels only poorly correlated with the pathological alterations found in the nephrohistological preparations.Citation[25] An obvious step was therefore to assess the value of measuring sICAM-1 in urine among patients with acute kidney failure. A study by Bechtel et al. in 1994 reported a very good correlation in this regard.Citation[26]
In our study, the sICAM-1 levels in urine over the entire hospitalization period remained significantly higher in the group with acute kidney failure than was the case in the control group (see ), whereby the values measured in the control group were higher than published values for healthy subjects and patients with later rejection reactions after kidney transplantation.Citation[11]
The concentration of sICAM-1 in urine, unlike the quantity of membrane bound ICAM-1, did not apparently depend on stimulation by IL-6, and was as such no direct expression of an endothelial inflammatory reaction. Because tubular epithelial cells are very stable in urine, an increased release of sICAM-1 from destroyed, ICAM-1-bearing epithelial cells is rather unlikely. An inflammation-independent endothelial dysfunction seems to exist at the onset of post-ischemic kidney damage. As such, although an increased expression of ICAM-1 and passage of sICAM-1 in the urine is shown in the area of the peritubular capillary plexus, there was in fact relatively little cellular destruction to be seen during this phase in the region of the proximal tubular epithelium.
sICAM-1 in the urine, in contrast to its high informative value as an early predicator of a predisposition toward threatening kidney failure, appears to be less suitable as a renal functional parameter, as no major changes in its concentration can be shown around the day of the clinical onset of acute kidney failure (see ), as was also suggested previously.Citation[11]
sE-Selectin is expressed temporarily as a cell surface glycoprotein on endothelial cells after stimulation by cytokines.Citation[12] Because sE-Selectin in the area of the kidney epithelium is expressed only upon damage, it is conceivable that a raised sE-Selectin level in urine may act as an early indicator for kidney damage.Citation[13]
In the present study, its suitability as a renal functional parameter could be confirmed. Suddenly and significantly higher values for sE-Selectin could be found in the urine a day before the increase in serum creatinine (T-1) in the group that developed acute kidney failure compared to the control group. After completion of the acute phase, these values fell off just as rapidly (see ). Because the sE-Selectin levels measured in plasma at the same time showed no such variations, it can be assumed that the cause did indeed lie in the increased expression of sE-Selectin on kidney endothelial cells and was not an indicator for a general systemic increase. In analogy to the findings with sICAM-1, the proximal tubular section can be assumed to be the location with the largest concentration, as sE-Selectin and ICAM-1 act synergistically.Citation[27]
sE-Selectin does not appear to be suitable as an early predicator for threatening kidney failure. No significant difference between the two groups could be found on the day after admission, so that, unlike sICAM-1, no assignment of the patients to a group with higher or lower risk for the development of acute kidney failure could be carried out.
Neither sICAM-1 nor sE-Selectin performed the task to recognize early and exactly kidney dysfunctions. For early detection of tubular damage, α1-MG, and NAG and for glomerular structure, total protein should be examined.
It has been shown that ICAM-1-deficient mice are resistant towards post-ischemic kidney failure.Citation[28] Novel therapeutic approaches, such as the administration of antibodies against ICAM-1 or therapy with recombinant sICAM-1, for postoperative and post-traumatic intensive care patients could be an effective treatment for acute renal failure, whereby the urinary concentration of sICAM-1 could be used as an indicator for the renal disorder.
sE-Selectin-deficient mice reveal a good recovery of renal function, while half of the normal, non-deficient mice develop an acute kidney failure. Mice treated with an antibody against sE-Selectin also show a very good recovery of renal function. This suggests that the prophylactic application of antibodies toward sE-Selectin after renal ischemia might prevent the origin of acute kidney failure in man as well, thus offering a potential therapeutic approach for this condition, monitored by the urinary excretion rate of sE-Selectin.Citation[29]
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Byrick RJ, Rose DK. Pathophysiology and prevention of acute renal failure: The role of the anaesthesist. Can J Anaesth. 1990; 37: 457–467
- Lynn KL, Marshall RD. Excretion of Tamm-Horsfall glycoprotein in renal disease. Clin Nephrol. 1984; 22: 253–257
- Itoh Y, Kawai T. Human alpha 1-microglobulin: Its measurement and clinical significance. J Clin Lab Anal. 1990; 4: 376–384
- Wedeen RP, Udasin I, Fiedler N. Urinary biomarkers as indicators of renal disease. Ren Fail. 1999; 21: 241–249
- Hotta O, Sugai H, Kitamura H, Yusa N, Taguma Y. Predictive value of urinary micro-cholesterol (mCHO) levels in patients with progressive glomerular disease. Kidney Int. 2004; 66: 2374–2381
- Higuchi H, Adachi Y. Renal function in surgical patients after administration of low-flow sevoflurane and amikacin. J Anesth. 2002; 16: 17–22
- Adams DH, Nash GB. Disturbance of leukocyte circulation and adhesion to the endothelium as factors in circulatory pathology. Br J Anaesth. 1996; 77: 17–31
- Adams DH, Hutchinson IV. Microchimerism and graft intolerance: Cause or effect?. Lancet. 1997; 349: 1336–1337
- Hogg N, Landis RC. Adhesion molecules in cell interactions. Curr Opin Immunol. 1993; 5: 383–390
- Gobé Glenda C, Willgoss D, Hogg N, Schoch E, Endre Z. Cell survival or death in renal tubular epithelium after ischemia-reperfusion injury. Kidney Int. 1999; 56: 1299–130
- Teppo AM, von Willebrand E, Honkanen E, Ahonen J, Gronhagen-Riska C. Soluble intercellular adhesion molecule-1 (sICAM-1) after kidney transplantation: The origin and role of urinary sICAM-1. Transplantation. 2001; 71(8)1113–1119
- Bevilacqua MP, Stengelin S, Gimbrone MA, Jr, Seed B. Endothelial leukocyte adhesion molecule 1: An inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989; 243: 1160–1164
- Gearing AJH, Hemingway I, Pigott R, Hughes J, Rees AJ, Cashman SJ. Soluble forms of vascular adhesion molecules, E-selectin, ICAM-1, and VCAM-1: Pathological significance. Ann NY Acad Sci. 1992; 667: 324–331
- Stewart Mairi P, Cabañas C, Hogg N. T cell adhesion to intercellular adhesion molecule-1 (ICAM-1) is controlled by cell spreading and the activation of integrin LFA-1. J Immunol. 1996; 156: 1810–1817
- Janssen BA, Luqmani RA, Gordon C, Hemingway I, Bacon PA, Gearing AJH, Emery P. Correlation of blood levels of soluble vascular cell adhesion molecule-1 with disease activity in systemic lupus erythematosus and vasculitis. Br J Rheumatol. 1994; 33: 1112–1116
- Dati F, Lammers M. Immunochemical methods for determination of urinary proteins in kidney disease. J Fed Clin Chem. 1989; 1: 68–77
- Simane ZJ, N-Acetyl-b-D-Glucosaminidase. In. Urinary Enzymes in Clinical and Experimental Medicine, K Jung, H Mattenheimer, U Burchardt. Springer, Berlin 1992; 118–124
- Sural S, Sharma RK, Singhal M, et al. Etiology, prognosis, and outcome of post-operative acute renal failure. Ren Fail. 2000; 1: 87–97
- Hartl WH, Jauch KW. Post-aggression metabolism: Attempt at a status determination. Infusionstherapie Transfusionsmedizin. 1994; 2: 30–40
- Rebel W, Bertsch T, Bode G, . Enzymuria as an indicator of renal pathomorphology. Urinary Enzymes in Clinical and Experimental Medicine, K Jung, H Mattenheimer, U Burchardt, et al. Springer, Berlin 1992; 43–72
- McAuley FT, Simpson JG, Thomson AW, Whiting PH. The predictive value of enzymuria in cyclosporin A-induced renal toxicity in the rat. Toxicol Lett. 1986; 32: 163–169
- Donadio C, Puccini R, Lucchesi A, Giordani R, Rizzo G. Urinary excretion of proteins and tubular enzymes in renal transplant patients. Ren Fail. 1998; 20: 707–715
- Wilkes BM, Mailloux LU. Acute renal failure. Pathogenesis and prevention. Am J Med. 1986; 80: 1129–1136
- Gauer S, Yao J, Schoecklmann HO, Sterzel RB. Adhesion molecules in the glomerular mesangium. Kidney Int. 1997; 51: 1447–1453
- Rothlein R, Kennedy C, Czajkowski M, Barton RW. Generation and characterization of an anti-idiotypic antibody specific for intercellular adhesion molecule-1. Int Arch Allergy Immunol. 1993; 100: 121–127
- Bechtel U, Scheuer R, Landgraf R, Konig A, Feucht HE. Assessment of soluble adhesion molecules (sICAM-1, sVCAM-1, sELAM-1) and complement cleavage products (sC4d, sC5b-9) in urine. Clinical monitoring of renal allograft recipients. Transplantation. 1994; 58(8)905–911
- Gearing AJH, Newman W. Circulating adhesion molecules in disease. Immunology Today. 1993; 14: 506–512
- Kelly K J, Williams WW, Jr, Colvin RB, Meehan SM, Springer TA, Gutierrez-Ramos JC, Bonventre JV. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Invest. 1996; 97: 1056–1062
- Singbartl K, Ley K. Protection from ischemia-reperfusion induced severe acute renal failure by blocking E-Selectin. Crit Care Med. 2000; 28(7)2507–2514