Abstract
There are few studies on the relationship between the morphology of acute tubular necrosis (ATN) in native kidneys and late functional recovery. Eighteen patients with acute renal failure (ARF) who had undergone renal biopsy were studied. All had the histological diagnosis of ATN and were followed for at least six months. Clinical characteristics of ARF were analyzed, and histological features were semi-quantitatively evaluated (tubular atrophy, interstitial inflammatory infiltrate, interstitial fibrosis, and ATN). According to the maximal GFR achieved during the follow-up, patients were divided into two groups: complete recovery (GFR ≥ 90 mL/min/1.73 m2) and partial recovery (GFR < 90 mL/min/1.73 m2). Only 39% of the patients achieved complete recovery. Patients with partial recovery achieved their maximal GFR (63 ± 9 mL/min/1.73 m2) 37 ± 14 months after ARF, a period of time similar to those patients with complete recovery (i.e., 54 ± 22 months). Patients with partial recovery had more severe ARF: oliguria was more frequent (90 versus 17%, p < 0.01), and they had higher peak creatinine (13.85 ± 1.12 versus 8.95 ± 1.30 mg/dL, p = 0.01), and longer hospitalization (45 ± 7 versus 20 ± 4 days, p = 0.03). No single histological parameter was associated with partial recovery, but the sum of all was when expressed as an injury index [4.00 (2.73–5.45) versus 2.00 (1.25–3.31), p < 0.05]. In conclusion, among patients with atypical ATN course, those with more severe ARF and tubule-interstitial lesions are more prone to partial recovery.
INTRODUCTION
Acute tubular necrosis (ATN) is the most important and frequent cause of acute renal failure (ARF). Although ATN is a histological finding, it is usually diagnosed only on clinical bases. Renal biopsy is performed in ARF when its cause is suspected to not be ATN. The Spanish Register of Glomerulonephritis showed ARF as an indication for the renal biopsy in 12.9% of the cases. Moreover ATN was diagnosed in fewer than 10% of these patients.Citation[1] Such an observation can explain why there are so few morphologic studies on ATN unrelated to kidney transplantation.
Many studies in renal transplantation have shown a relationship between ATN diagnosed by biopsy of the graft and late renal function. However, there are very few in human native kidneys. Bonomini et al. reported that one year after histologically proven ATN, 62.4% of the patients had complete functional recovery (GFR > 80 mL/min), 31.2% had partial recovery, and 6.4% had end stage renal disease (ESRD). The same patients evaluated five years after ATN showed that only 56.8% remained with complete recovery and 11.2% had end stage renal disease (ESRD).Citation[2] In humans, persistent alterations in tubular function, as impaired urinary concentration and/or acidification, have been reported after an episode of ARF, even in patients with complete GFR recovery.Citation[3],Citation[4] Also in animals, impaired urinary concentration can persist after GFR restoration. Rats that presented normal GFR and fractional excretion of sodium 40 weeks after 60 minute bilateral ischemia showed impaired urinary concentration. In these animals, the finding of reduced peritubular capillaries in the inner stripe of outer medulla and of tubulointerstitial fibrosis could justify the observed functional defect.Citation[5] Other experimental studies of ARF have also shown that chronic structural changes may be observed after the acute injury.Citation[6],Citation[7]
In the 1970s, Striker et al. pointed to the association between interstitial disease and decline of GFR.Citation[8] A more recent study on ATN after renal transplantation showed that patients who had progressed to chronic renal failure presented larger fractional interstitial area in their biopsies than patients with good outcome (34% versus 19%, p < 0.01). One woman with ATN in native kidneys who progressed to ESRD also had a large fractional interstitial area in her biopsy (i.e., 36%).Citation[9] However, the presence and intensity of interstitial fibrosis, tubular atrophy, or cellular interstitial infiltrate have not been studied as prognostic factors of late functional recovery in human native ATN. This is the aim of the present study.
PATIENTS AND METHODS
Patients
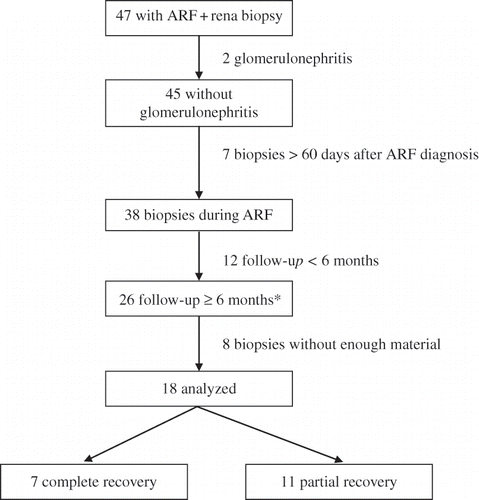
From 1985 to 2005, our group attended to 47 patients with ARF unrelated to renal transplantation who underwent renal biopsy. All biopsies had been performed by puncture, guided or not by ultrasound, up to 60 days after the ARF diagnosis. Two were excluded from the study because the histological diagnosis was glomerulonephritis and seven because renal biopsy was performed more than 60 days after hospital discharge. No patient was discharged requiring dialysis. All remaining 38 patients had the histological diagnosis of ATN and were referred to our outpatient clinic after the hospital discharge. Twelve left the follow-up before six months. Among the remaining 26 patients, only 18 had enough material to be analyzed, and they composed the studied sample. Their biopsies were reviewed by one of the authors (DMACM) in a semi-quantitative way. No patient had any evidence of previous renal dysfunction. The flow chart of patients' selection is presented in .
Histomorphometric Evaluation
Morphometric evaluations were performed in a blinded manner by a single observer (DMACM). The presence of tubular atrophy, interstitial inflammatory infiltrate, and ATN was estimated in 4 μm-thick sections stained with periodic acid-Schiff. The extent of interstitial fibrosis was estimated in Masson-stained slides. Each histologic parameter was evaluated examining all microscopic fields of each slide, at a final magnification of 250 ×. For each parameter, a score was attributed as follows:
0: <5% of the section affected;
1: 6–25% of the section affected;
2: 26–50% of the section affected;
3: >50% of the section affected.Citation[10]
An injury index was calculated as the sum of the scores obtained for these four evaluated parameters.Citation[10] Atherosclerotic alterations were evaluated and graded as present or absent, as well as the percentage of sclerotic glomeruli relative to total number of glomeruli in the slice. At least three glomeruli were examined in each slice.
Acute Renal Failure Characteristics
We retrospectively analyzed ARF characteristics: patient's age and gender, presence of co-morbidities (diabetes mellitus, hypertension), presence of oliguria (urinary volume < 400 mL/24 h after appropriated hydration), need for dialysis, period of time between ARF diagnosis and renal biopsy (days), indication for renal biopsy (course of ARF unduly prolonged, clinical presentation atypical for ATN, and suspicion of acute allergic interstitial nephritis), peak plasma creatinine (Pcreat), and Pcreat at hospital discharge. We also analyzed the period of time when the patient had ARF (i.e., from 1986 to 1990 and from 1991 to 2005).
Follow-Up
All the patients were followed-up by one of the authors (RCRMA). Usually the patient was seen as an outpatient 1–2 weeks after the hospital discharge, every three months afterward until Pcreat stabilized, and from then every 6–12 months (or more frequently, if necessary) until death, ESRD, or referral to another service due to the development of disease that needed specialized treatment. Some patients left the follow-up on their own. In each consultation, Pcreat was measured. We retrospectively analyzed the minimum Pcreat value during the follow-up, time to reach this value, and the last Pcreat value, as well as when it was measured. For each Pcreat value, GFR was estimated using the abbreviated Modification of Diet Renal Disease (MDRD) equation.Citation[11]
Statistics
In order to evaluate the factors influencing renal function recovery, the patients were divided in two groups: complete recovery (n = 7, maximal GFR ≥ 90 mL/min per 1.73 m2) and partial recovery (n = 11, maximal GFR < 90 mL/min per 1.73 m2). This cutoff value was chosen because it is the limit of normal GFR considered in the K/DOQI classification of chronic kidney disease.Citation[11] Student's t test or Mann-Whitney test were used as appropriate to compare continuous variables. Chi-square or Fischer's exact test were used for qualitative data. All statistical analysis was performed using GraphPad Prism statistical program, version 4.0 for Windows (GraphPad Software, San Diego, California, USA). Values of p < 0.05 were considered statistically significant. Data are presented as mean ± SEM, median (25–75 percentile), or percentage.
RESULTS
Eighteen patients (12 males and 6 females) aged 45 ± 9 years (17–69 years) at the ARF episode were followed-up for 13 to 250 months. Renal biopsy showed ATN in all of them.
ARF Characteristics
Only 7 of the 18 studied patients achieved complete recovery (39%). Only one patient (with partial recovery) had diabetes, and two patients (with complete recovery) had leprosy. The frequency of hypertension is shown in . The patients with complete recovery, as shown in , had less severe ARF (i.e., less frequent oliguria, lower peak Pcreat, and shorter hospitalization). Four patients with complete recovery had ARF in the period 1986–1990 and three in the period 1991–2005; a similar distribution was found among the patients with partial recovery (respectively 5 and 6 patients in each period, p = 1.00). Maximal GFR was achieved more than three years after the hospital discharge in both groups, as seen in . Fifteen patients achieved their maximal GFR before the last measured GFR. Once these 15 patients had achieved their maximal GFR, a slight but significant decrease was observed. However, the rate of GFR decline was similar in both groups (p = 0.3153).
Table 1 Patient demographics and acute renal failure variables (mean ± SEM)
Table 2 Follow-up data
Histological Features
The indication for the biopsy was atypical ATN in 10 patients, prolonged ARF in 5, and suspicion of allergic acute interstitial nephritis in 3 (see ). This distribution was similar in both groups (p = 0.0966). Each single histological parameter had a similar value in both groups (see ). However, the injury index value was higher in the patients with partial recovery, 4.0 (2.73–5.45) versus 2.0 (1.25–3.31), p = 0.0474.
Table 3 Clinical characteristics of all studied patients
Table 4 Histological features
DISCUSSION
Renal biopsy is an important tool in diagnosing the cause of renal dysfunction. In glomerular diseases, the morphologic alterations disclose the diagnosis, and the grade of interstitial fibrosis or the number of crescents and their characteristics point to the prognosis. However, in native kidneys, the value of ATN finding has been limited to diagnosis. The role of ATN characteristics in determining functional prognosis is unknown. Among our patients, no single histological parameter was associated with partial recovery, but the sum of all tubulointerstitial alterations (i.e., tubular atrophy, interstitial fibrosis, interstitial inflammatory infiltrate, and ATN) was. In contrast, in renal transplantation, the intensity of tubular necrosis in donor renal biopsy has been associated with delayed function of the graft. The intensity of the expression of poly(ADP-ribose) polymerase (PARP-1) in tubular cells (i.e., the activation of PARP-1 in conditions of tissue ischemia results in cell necrosis) was associated with time to effective diuresis (r = 0.386, p = 0.01), Pcreat three months after transplantation (r = 0.374, p = 0.013), and donor age (r = 0.408, p = 0.006).Citation[12] Also in IgA nephropathy, Gutierrez et al. showed that among 32 patients who have had an episode of macroscopic hematuria-induced ARF, 25% of them had not returned to baseline Pcreat six months after the episode.Citation[13] These patients were older and had longer, but not more frequent, episodes of macroscopic hematuria and higher baseline Pcreat. Except for the intensity of tubular necrosis (more severe in these patients), the other morphologic changes observed in renal biopsy (i.e., mesangial proliferation, global glomerulosclerosis, percentage of glomeruli with crescents, percentage of tubules filled with red blood cell casts, interstitial fibrosis) were similar to those found in the patients that returned to baseline Pcreat.Citation[13] The discrepancy between our results and those cited can be explained by the time of renal biopsy (i.e., very early in the first study and ∼3 weeks in ours) and the cause of ARF (i.e., unifactorial in both cited studies and multifactorial or unusual).
Different causes of ARF can induce different histological alterations. Some Chinese herbs that contain aristolochic acid (AA) can induce ATN without functional recovery. Yang et al. compared the histomorphologic alterations found in renal biopsy of eight patients with AA-induced ATN with the alterations found in nine patients with antibiotic-induced ATN.Citation[10] Both groups had a similar age, gender, time of drug exposition, and Pcreat at the time of renal biopsy. In the 2–6-month follow-up, all patients with antibiotic-induced ARF had their Pcreat decrease at least 50% or normalize, while all patients with AA-induced ATN presented no changes or even worsened in Pcreat. The intensity of ATN was similar in both groups and related to Pcreat at the time of renal biopsy. However, there was active tubule cell proliferation in antibiotic-induced ATN, in sharp contrast with almost no proliferating tubular epithelial cells in AA-induced ATN. Interstitial extracellular matrix deposition was detected only in AA-induced ATN despite the expression of beta1-transforming growth factor and connecting tissue growth factor found to be increased in both groups. The peritubular capillaries density was slightly diminished in antibiotic-induced ATN but clearly reduced in AA-induced ATN. Also, in this last group, the structure of endothelial cells was altered. There was a strong correlation between VEGF expression in renal tubules and either peritubular capillary density or tubular proliferation. The authors concluded that AA leads to peritubular capillary loss and consequent diminished cellular regeneration and tendency to fibrosis. Also in different experimental models, as ischemia, gentamicin, or glycerol, interstitial fibrosis can progress even after functional recovery.Citation[7],Citation[14],Citation[15]
When renal biopsy is performed in native kidney ARF, the histological findings mirror the consequence of an acute injury as tubular necrosis and the response to this acute injury, such as cellular regeneration, inflammatory infiltrate, progressive fibrosis, or loss of glomeruli; however, they also mirror chronic features (present before ARF) such as sclerosed glomeruli and atherosclerotic vascular alterations. Thus, the finding of more intense histological abnormalities in the group with partial recovery represents the result of more severe ARF but also of aging, chronic hypertension, etc. Aging can decrease renal reserve and worsen functional recovery after an ischemic injury. In rats, it has been shown that older animals had more senescent cells (senescence evaluated by the intensity of beta-galactosidase staining) and worse renal recovery after a 45-min ischemic injury.Citation[16]
Our result—that is, only 39% of patients with complete recovery—is not in accordance with the better evolution recently described by Schiffl in patients with ARF (supposed to be ATN) requiring RRT but with normal baseline Pcreat.Citation[17] The author showed that at hospital discharge, 57% of the surviving patients had normal Pcreat, 33% had Pcreat between 1.3 and 3 mg/dL, and 10% had Pcreat from 3 to 6 mg/dL. At hospital discharge, no patient needed dialysis. Functional recovery was not related to patients' characteristics, severity of disease, or type and duration of dialysis. At one year, 34% of these patients had died, and one required chronic dialysis.Citation[17] However, a recent study of 1,102 patients who needed RRT during ARF showed that 104 (9.4%) were on chronic dialysis within 90 days after the first dialysis, and a further 34 developed a late need for chronic dialysis.Citation[18] Our patients cannot be compared with patients with usual ARF: our patients had prolonged or atypical ARF, even with biopsy-proven ATN. Moreover, we defined functional recovery by normal GFR not by a normal Pcreat, which is a bad marker for renal dysfunction, or by the independence from dialysis, which is a very specific but surely not sensitive marker of partial functional recovery.
In accordance with our data suggesting that the severity of ARF can influence renal function recovery, Ali et al., in a population-based study, showed that full renal recovery at 90 days was associated with less ARF severity, evaluated according to RIFLE classification (i.e., risk of renal failure, injury to the kidney, failure of kidney function, loss of kidney function, and end-stage renal failure). They found a 71% full recovery rate among patients classified as Risk, 75% among those classified as Injury, but only 51% among those classified as Failure, p < 0.001.Citation[19]
Our findings point to the importance of renal biopsy in patients with prolonged or atypical ARF. Also, in native kidneys, the quantification of the histological severity of ATN, besides confirming the correct diagnosis, can anticipate functional evolution. Other studies on the markers of renal function recovery after ATN are warranted, but renal biopsy is a very important tool.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
ACKNOWLEDGMENT
Part of the results was presented as poster in Renal Week 2006 (Libório AB, Vieira Jr JM, Malheiros DM, Abdulkader RCRM. Histological findings in acute tubular necrosis do not predict long term renal function. Journal of the American Society of Nephrology 17: 284A, abstract TH-PO834, 2006).
REFERENCES
- Rivera F, López-Gómez JM, Pérez-García R, on the behalf of the Spanish Registry of Glomerulonephritis. Clinicopathologic correlations of renal pathology in Spain. Kidney Int. 2004; 66: 898–904
- Bonomini V, Stefoni S, Vangelista A. Long-term patient and renal prognosis in acute kidney injury. Nephron. 1984; 36: 169–172
- Abdulkader R, Malheiro P, Daher E, et al. Late evaluation of glomerular filtration rate, proteinuria, and urinary acidification after acute tubular necrosis. Ren Fail. 1992; 14: 57–61
- Finn WF. Recovery from acute renal failure. Acute Renal Failure, JM Lazarus, BM Brenner. Churchill Livingstone, New York 1993; 553–596
- Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Ren Physiol. 2001; 281: F887–F899
- Forbes JM, Hewitson TD, Becker GJ, Jones CL. Ischemic acute kidney injury: Long-term histology of cell and matrix changes in the rat. Kidney Int. 2000; 57: 2375–2385
- Soares TJ, Costa RS, Volpini RA, da Silva CG, Coimbra TM. Long-term evolution of the acute tubular necrosis (ATN) induced by glycerol: Role of myoblasts and macrophages. Int J Exp Pathol. 2002; 83: 165–172
- Striker GE, Quadracci LJ, Cutler RE. Use and interpretation of renal biopsy. Major Problems in Pathology, JL Bennington. W.B. Saunders, Philadelphia 1978; 1–347
- Moyses-Neto M, Costa RS, Volpini RA, Garcia TMP, Rodrigues FF, Coimbra TM. Interstitial alterations in renal cortex in acute tubular necrosis (ATN) post-renal transplantation and in patients with ATN not related to renal transplantation. Clin Transplant. 2004; 18: 156–165
- Yang L, Li X, Wang H. Possible mechanisms explaining the tendency towards interstitial fibrosis in aristolochic acid-induced acute tubular necrosis. Nephrol Dial Transplant. 2007; 22: 445–456
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002; 39(Suppl. 1)S1–S266
- O'Valle F, Benitez MC, Gómez-Morales M, et al. Correlation of morphological findings with functional reserve in aging donor: Role of poly(ADP-ribose) polymerase. Transplant Proc. 2004; 36: 733–735
- Gutiérrez E, González E, Hernández E, et al. Factors that determine an incomplete recovery of renal function in macrohematuria-induced acute renal failure of IgA nephropathy. Clin J Am Soc Nephrol. 2007; 2: 51–57
- Pagtalunan ME, Olson JL, Tilney NL, Meyer TW. Late consequences of acute ischemic injury to a solitary kidney. J Am Soc Nephrol. 1999; 10: 366–373
- Geleilete TJ, Melo GC, Costa RS, Volpini RA, Soares TJ, Coimbra TM. Role of myoblasts, macrophages, transforming growth factor-beta, endothelin, angiotensin II, and fibronectin in the progression of tubulointerstitial nephritis induced by gentamicin. J Nephrol. 2002; 15: 633–642
- Chkhotua A, Shapira Z, Tovar A, Shabtai E, Yussim A. Cellular senescence: A new marker of kidney function recovery after ischemic injury in rats. Transplant Proc. 2001; 33: 2910–2915
- Schiffl H. Renal recovery from acute tubular necrosis requiring renal replacement therapy: A prospective study in critically ill patients. Nephrol Dial Transplant. 2006; 21: 1248–1252
- Bell M, NG SWI, Granath F, Schon S, Ekbom A, Martling CR. Continuous renal replacement therapy is associated with less chronic renal failure than intermittent haemodialysis after acute renal failure. Intensive Care Med. 2007; 33: 773–780
- Ali T, Khan I, Simpson W, et al. Incidence and outcomes in acute kidney injury: A comprehensive population-based study. J Am Soc Nephrol. 2007; 18: 1292–1298