Abstract
Introduction. Nitric oxide (NO) and peroxynitrite (OONO—) are implicated in the pathophysiology of renal ischemia/reperfusion (I/R). The aim of this study was to investigate and compare the efficiency of S-methylisothiourea (SMT), an iNOS inhibitor, and mercaptoethylguanidine (MEG), a scavenger of peroxynitrite, on renal dysfunction and injury induced by I/R of rat kidney. Materials and Methods. Thirty-two male Sprague-Dawley rats were divided into four groups: sham-operated, I/R, I/R+SMT, and I/R+MEG. Rats were given SMT (10 mg/kg ip) or MEG (10 mg/kg ip) 6 h prior to I/R and at the beginning of reperfusion. All rats except sham-operated underwent 60 min of bilateral renal ischemia followed by 6 h of reperfusion. After reperfusion, kidneys and blood were obtained for evaluation. Superoxide dismutase, glutathione peroxidase, malondialdehide, protein carbonyl content, and nitrite/nitrate level (NOx) were determined in the renal tissue. Serum creatinine (SCr), blood urea nitrogen (BUN), and aspartate aminotransferase (AST) were determined in the blood. Additionally, renal sections were used for histological grade of renal injury. Results. SMT and MEG significantly reduced the I/R-induced increases in SCr, BUN, and AST. Both SMT and MEG attenuated the tissue NOx levels, indicating reduced NO production. In addition, SMT and MEG markedly reduced elevated oxidative stress product, restored decreased antioxidant enzymes, and attenuated histological alterations. Interestingly, MEG exerted a greater renoprotective effect than SMT. Conclusions. These data support the finding that iNOS and peroxynitrite are involved in the renal I/R injury, and suggest that a scavenger of peroxynitrite might be more effective than iNOS inhibitors as a therapeutic intervention.
INTRODUCTION
Ischemia/reperfusion (I/R) injury in the kidney leads to acute renal failure (ARF), which occurs in the transplantation, shock, sepsis, and renal artery stenosis. Despite significant advances in critical care medicine, ARF is still a major clinical problem associated with considerable morbidity and mortality, which has not decreased significantly over the last 60 years.Citation[1],Citation[2] The pathophysiology underlying the development of ARF is complex and not yet fully elucidated. Although pharmacological interventions have been studied in several experiments, none of these have gained popularity in clinical studies and dialysis, and transplantation remains the current therapeutic intervention for ARF.Citation[1] Therefore, the development of therapeutic interventions to prevent or reduce renal tissue injury after I/R remains the focus of research.
Reperfusion of tissue or the kidney after ischemic period causes the release of proinflammatory substances and the formation of both nitrogen-derived (reactive nitrogen species [RNS]) and oxygen-derived (reactive oxygen species [ROS]) free radicals, such as superoxide (O2—), peroxide (H2O2) and hydroxyl radicals (OH—).Citation[2–4] In addition to these radicals, nitric oxide (NO) synthesized by inducible nitric oxide (iNOS) is also important. NO may react with O2— to generate potent oxidant peroxynitrite (ONOO—), which is capable of nitrating tyrosine residues of proteins and enzymes.Citation[5–7] These free radicals are normally removed by antioxidant enzymes, mainly superoxide dismutase (SOD) and gluthationeperoxidase (GPx). I/R injury can be evaluated by the detection of various products resulting from injury, using a laboratory and histomorphological methods. Malondialdehyde (MDA) frequently used to show the involvement of free radicals in cell damage is one of the final products of lipid peroxidation. Besides lipid peroxidation, the measurement of protein damage by protein carbonyl content (PCC) and antioxidants enzyme activities can be performed to quantify ROS/RNS in laboratory.Citation[8–11]
Various in vivo studies have shown that NO biosynthesis and its action are closely related to the pathogenesis of renal I/R injury.Citation[7],Citation[12] Several investigations have shown that selective or nonselective iNOS inhibitors can prevent I/R injury in the kidney.Citation[5],Citation[7],Citation[13] Moreover, these studies suggest that renal injury may be mediated by ONOO—. Although iNOS inhibitors in renal I/R have been studied extensively, there is only one report related to ONOO— inhibition in renal I/R that demonstrates that ebselen, a scavenger of ONOO—, improves renal function subject to I/R due to the suppression of ONOO— production or its scavenging.Citation[14]
Mercaptoethylguanidine (MEG) is a scavenger of peroxynitrite.Citation[15] It was shown that MEG has a dramatic protective effect in many experimental models of inflammation, including periodontitis,Citation[6] hemorrhagic shock,Citation[16] inflammatory bowel disease,Citation[17] and endotoxic and septic shock.Citation[16] In addition, S-methylisothiourea (SMT) is a more potent inhibitor for iNOS, and it was reported that SMT increases survival rate in rats with endotoxic shock and bacterial peritonitis.Citation[18]
Based on these reports implicating iNOS, NO, and ONOO— in ischemic ARF, this study was designed to determine whether pharmacological inhibition of iNOS or scavenging of ONOO? has protective potential in renal I/R injury. Thereby, the potential influence of MEG and SMT on renal antioxidant enzyme activity, tissue damage, and histopathological changes was investigated.
MATERIALS AND METHODS
The project was approved by the Experimental Ethics Committee of Gulhane Military Medical Academy, Ankara, Turkey, and the National Institute of Health's Guide for the Care and Use of Laboratory Animals was followed.
Surgery and Experimental Protocol
Thirty-two male Sprague-Dawley rats weighing 250–300 g were provided by the Gulhane Military Medical Academy, Experimental Research Council, and housed in standard cages at a constant temperature (24°C), humidity (70%), and light-dark cycle in a controlled environment. Rats were under standard rat chow and water ad libitum.
Rats were randomly divided into four groups: sham-operated (n = 8), renal I/R (n = 8), renal I/R+SMT (n = 8), and renal+MEG (n = 8). Following a 12 h fasting period, animals were anesthetized with an intraperitoneal injection of ketamine hydrochloride (50 mg/kg) and xylazine (10 mg/kg). The rats were placed on a heating pad kept at 39°C to maintain constant body temperature. A midline incision was made, the renal pedicle observed, and arteries bilaterally occluded with an atraumatic microvascular clamp (Vascustatts II, midi 1001–532, Scatlan, Minnesota, USA) for 60 min. The time of ischemia was chosen to maximize the reproducibility of renal functional impairment while minimizing mortality in these animals. After 60 min of renal ischemia, clamps were removed and the kidneys were inspected for restoration of blood flow. The abdomen was closed in two layers. Sham-operated animals underwent the same surgical procedure without clamp application. Following 6 h of reperfusion period, animals were killed by cervical dislocation. At the time of death, blood was collected by heart puncture for measurement of biochemical analysis. Both kidneys were harvested for histopathological evaluation and biochemical examination.
Biochemical Analysis
Biochemical Parameters
Serum samples were used for the measurement of blood urea nitrogen (BUN) and serum creatinine (SCr) levels, which were used as indicators of impaired glomerular function, and aspartate aminotransferase (AST), which was used as an indicator of renal I/R injury.Citation[5] BUN, SCr, and AST were determined with an Abbott-Aeroset autoanalyzer (Chicago, Illinois, USA) using original kits.
Tissue Preparation
The frozen tissues were homogenized in phosphate buffer (pH 7.4) by means of a homogenizator (Heidolph Diax 900; Heidolph Elektro GmbH, Kelhaim, Germany) kept on ice. The supernatant was stored at −70°C.
Proteins Assay
Initially, the protein content of tissue homogenates was measured by the method of Lowry et al.Citation[19] with bovine serum albumin as the standard.
Determination of Tissue Protein Carbonyl Content (PCC)
The tissue PCC was determined spectrophotometrically by the method based on the reaction of the carbonyl group with 2, 4-dinitrophenylhydrazine to form 2, 4-dinitrophenylhydrazine.Citation[20] 2, 4-Dinitrophenylhydrazine was the reagent originally used for proteins subjected to metal-catalyzed oxidation. Absorbances were measured with a spectrophotometer (Helios, Epsilon, Massachusetts, USA). The results were given as millimoles carbonyl per gram protein.
Determination of Tissue Lipid Peroxidation Level (MDA)
Lipid peroxidation level was measured with the thiobarbituric acid (TBA) reaction using the method described by Ohkawa.Citation[21] This method was used to obtain a spectrophotometric measurement of the color produced during the reaction to thiobarbituric acid (TBA) with MDA at 535 nm. MDA levels were expressed as mmol/g protein.
Determination of Superoxide Dismutase Activity (SOD)
SOD activity was assayed using the nitroblue tetrazolium (NBT) method of Sun et al.Citation[22] NBT was reduced to blue formazan by O2—, which has a strong absorbance at 560 nm. One unit (U) of SOD is defined as the amount of protein that inhibits the rate of NBT reduction by 50%. The calculated SOD activity was expressed as U/gprotein.
Determination of Glutathione Peroxidase (GPx)
The GPx activity was measured using the method described by Paglia and ValentineCitation[23] in which GPx activity was coupled with the oxidation of NADPH by glutathione reductase. The oxidation of NADPH was spectrophotometrically followed up at 340 nm at 37°C. The absorbance at 340 nm was recorded for 5 min. The activity was the slope of the lines as mmol of NADPH oxidized per minute. GPx activity was presented as U/g protein.
Determination of Nitrate and Nitrite (NOx)
Nitrate and nitrite (NOx) activity was measured using the method described by Miranda et al.Citation[24] Tissue homogenates centrifuged at 2000 × g for 5 min. The supernatant (0.5 ml) was added to 0.25 ml 0.3 M NaOH. After incubation for 5 min at room temperature, 0.25 ml of 5% (w/v) ZnSO4 was added for deproteinization. This mixture was then centrifuged at 3000 × g for 20 min (+ 4°C), and the supernatants were used for the assays.
A nitrate standard solution (100 μl) was serially diluted (generally from 200–1.6 mM) in duplicate in a 96-well plate. After loading the plate with samples (100 μl), the addition of VCl3 (100 μl) to each well was rapidly followed by addition of the Griess reagents, sulfanilamide (SULF) (50 μl), and N-(1-Naphthyl)ethylenediamine (NEDD) (50 μl). The Griess solutions may also be premixed immediately prior to application to the plate. Sample blank values were obtained by substituting diluting medium for Griess reagent.
Nitrite was measured in a similar manner except that samples and nitrite standards were only exposed to Griess reagents. In either case, the absorbance at 540 nm was measured using a plate reader following incubation (30 min).
Tissue NOx levels were expressed as μmol/g tissue.
Histopathologic Evaluation
Tissue specimens were fixed in 10% formalin and embedded in paraffin and cut into 5 μm section. Slides were prepared with hematoxylin and eosin (H&S) stain and examined under light microscopy. Each slide was evaluated in a blind manner by two separate investigators for tubular cell swelling, brush border loss, nuclear condensation and nuclear loss. 100 intersections were examined for each kidney. Injury was graded on a four-tiered scale as follows: Grade 0 represent no diagnostic change; Grade 1 demonstrates tubular cell swelling, brush border loss, nuclear condensation with up to 1/3 of tubular profile showing nuclear loss; Grade 2 is similar to grade 1, but greater than 1/3 and less than 2/3 of tubular profile showing nuclear loss; Grade 3 displays greater than 2/3 of tubular profile shows nuclear loss. The total score for each kidney was calculated by the addition of all 100 scores with a maximum score of 300.Citation[5]
Statistical Analysis
All data are expressed as mean ± standard error of the mean (SEM). All statistical analyses were carried out using SPSS statistical software (SPSS for Windows, Version 15.0, Chicago, Illinois, USA). Differences in measured parameters among the three groups were analyzed by Kruskal-Wallis test. Dual comparisons between groups that present significant values were evaluated with Mann-Whitney U test. Statistical significance was accepted a value of p <0.05.
RESULTS
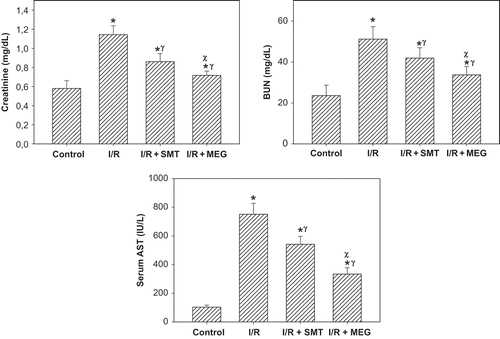
As shown in , rats that underwent renal I/R exhibited significant increases in the SCr and BUN levels compared to sham-operated group, suggesting a significant degree of glomerular dysfunction. Treatment of rats with SMT and MEG produced a slight, but statistically significant, reduction in the SCr and BUN levels compared to I/R group. Rats treated with MEG exhibited a statistically significant decrease in the SCr and BUN levels compared to those treated with SMT. Renal I/R produced a significant increase in the serum AST level in the I/R group. Serum concentration of AST, used as a marker of I/R injury, was significantly reduced in the rats treated with SMT and especially with MEG.
Figure 1. Effect of renal ischemia reperfusion (I/R), SMT, and MEG on serum creatinine, blood urea nitrogen (BUN), and serum aspartate aminotransferase (AST) levels. All values expressed as mean SEM. *statistically significant from control (p <0.05), γ = statistically significant from I/R group (p <0.05), χ = statistically significant from I/R+SMT (p <0.05).
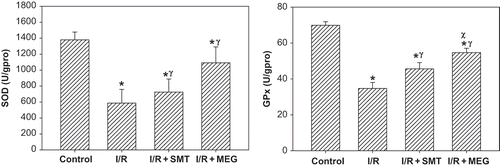
SOD and GPx enzyme activities are displayed in . Of all groups, the antioxidant enzymes activities were significantly lower in the I/R group (p <0.01). Although SOD activity in the treatment groups were significantly higher than the I/R group (p <0.05), it was significantly lower in the rats that received SMT compared to the sham-operated group (p <0.05). GPx activity was significantly higher in the I/R+SMT and I/R+MEG groups than the I/R group (p <0.05).
Figure 2. Effect of renal ischemia reperfusion (I/R), SMT, and MEG on tissue superoxide dismutase (SOD) and glutathione peroxidase (GPx) enzyme activities. All values expressed as mean SEM. *statistically significant from control (p <0.05), γ = statistically significant from I/R group (p <0.05), χ = statistically significant from I/R + SMT (p <0.05).
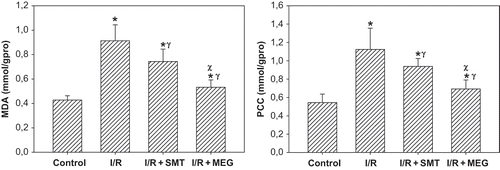
MDA level and PCC in the renal tissue are displayed in . The rats subjected to renal I/R revealed a strike increase in tissue MDA and PCC levels (p <0.01), suggesting increased lipid peroxidation and protein oxidation. The levels of MDA and PCC in the rats received SMT and MEG groups were lower than the I/R group (p <0.05).
Figure 3. Effect of renal ischemia reperfusion (I/R), SMT, and MEG on tissue malone dialdehyde (MDA) and protein carbonyl content (PCC). All values expressed as mean SEM. *statistically significant from control (p <0.05), γ = statistically significant from I/R group (p <0.05), χ = statistically significant from I/R+SMT (p <0.05).
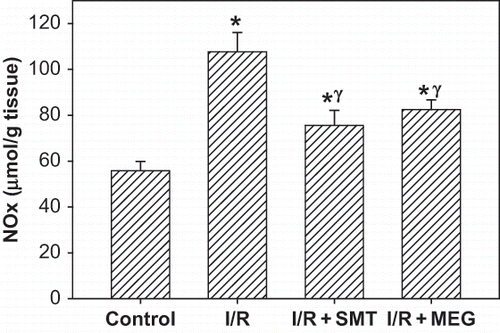
NOx level (nitrite/nitrite concentration) in the renal tissue, as indicator of NO synthesis, was significantly increased in the rats subjected to renal I/R (p <0.01). Increased tissue NOx levels were significantly decreased in the I/R+SMT and I/R+MEG groups (p <0.05). Tissue NOx levels in the I/R+SMT and I/R+MEG groups were higher than in the sham-operated group, and there was no statistically significance between treatment groups (see ).
Figure 4. Effect of renal ischemia reperfusion (I/R), SMT, and MEG on tissue nitrite/nitrate (NOx) level. All values expressed as mean SEM. *statistically significant from control (p <0.05), γ = statistically significant from I/R group (p <0.05), χ = statistically significant from I/R+SMT (p <0.05).
Histological grading of renal injury is displayed in . Renal I/R produced a significant increase in the total histological injury score, indicating significant tubular injury (p <0.01) when compared to score obtained from control group. Total histological injury score was significantly decreased in the rats receiving SMT and MEG. Interestingly, the total score of I/R+MEG group was significantly lower than I/R+SMT group (p <0.05). Representative histological samples from all groups are displayed in .
Figure 5. Effect of renal ischemia reperfusion (I/R), SMT, and MEG on the total renal histological injury score. All values expressed as mean SEM. *statistically significant from control (p <0.05), γ = statistically significant from I/R group (p <0.05), χ = statistically significant from I/R+SMT (p <0.05).
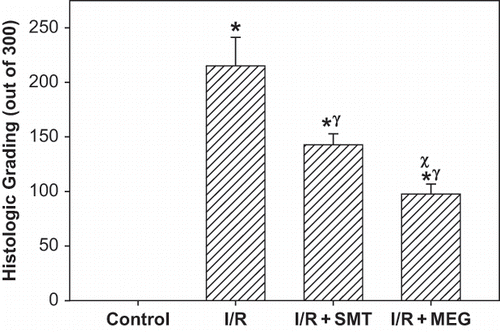
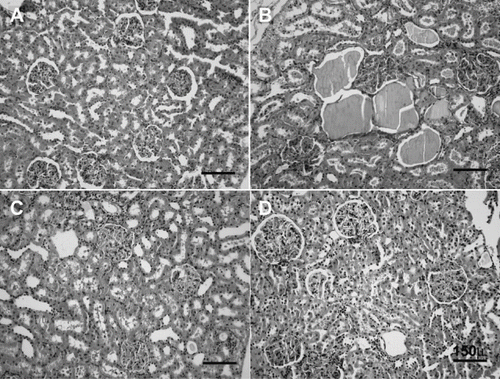
Figure 6. H&E stained of rat kidneys. A) Kidney section of sham-operated rat showing glomeruli and tubules appear normal. B) Kidney section of rat subjected to ischemia reperfusion (I/R) showing tubular cell swelling, nuclear condensation, and tubular dilatation. C) Kidney section of SMT-treated rat showing minimal tubular cell swelling, brush border loss, and nuclear condensation. D) Kidney section of MEG-treated rat showing minimal tubular cell swelling and nuclear condensation (hematoxylin and eosin; original magnification × 40).
DISCUSSION
The present study evaluated the effect of SMT, a potent inhibitor for iNOS, and MEG, a scavenger of peroxynitrite, in renal I/R injury. The results show that the treatment of renal I/R injury with SMT and MEG reduces renal glomerular and tubular dysfunction in rats. Histological examination of the kidneys subjected to I/R revealed lesions that were significantly decreased in animals treated with the SMT and MEG. In addition, MEG markedly ameliorated the antioxidant status and histological injury score of the kidneys. These results indicate that MEG improves the renal dysfunction and tissue injury induced by I/R, and that these effects are most likely accompanied by the scavenging of peroxynitrite, a proposed causal factor in the pathogenesis of I/R injury.
Renal I/R is one of the main causes of ARF, usually diagnosed after injury has already occurred. The aim of administration of drugs before injury occurred was to clarify a possible role for inhibition of iNOS and scavenger of peroxynitrite in renal I/R injury.
Renal I/R causes both glomerular and tubular dysfunction.Citation[2],Citation[3] We found that increased SCr and BUN levels subsequent to renal I/R indicate significant impairment of glomerular function. Renal I/R also produced a significant increase in the serum AST level, used as a marker of renal I/R injury in this study. Evidence of tubular injury was also supported by histological scoring of renal injury. Although both SMT and MEG produced a significant reduction of renal dysfunction and injury mediated by I/R of the kidneys, the degree of protection was higher in the rats that received MEG than in those that received SMT. It is likely that ONOO— is more involved in the renal I/R injury process than NO.
We found that antioxidant enzyme activities (SOD and GPx) and indices of tissue injury (MDA and PCC) were ameliorated in treatment groups. The generation of ROS and/or the decline of antioxidant defenses lead to oxidative stress, which plays an important role in the renal I/R injury and ARF.Citation[4] Under normal conditions, ROS can be scavenged by antioxidant enzymes such as SOD, which converts O2— to H2O2, and GPx, which breaks down H2O2 to H2O and O2. However, during renal I/R, excessive ROS generation occurs as demonstrated in many biochemical and immunohistochemical studies.Citation[3],Citation[25]
The accumulation of ROS and reduction in antioxidant enzyme activities lead to damage in cellular components such as lipids and proteins, resulting in an increase in MDA and PCC levels. Several investigations have demonstrated the beneficial effects of antioxidants, which prevent ROS generation and ROS scavenging molecules in renal I/R injury.Citation[3],Citation[25],Citation[26] Our observation, the amelioration of SOD and GPx enzyme activities and lipid peroxidation and protein oxidation, finds that MEG protects the kidney against I/R injury more effectively than SMT. The most possible explanations for these findings are that MEG has been proposed to inhibit cyclooxygenases, block the production of superoxide anions from activated leucocytes, and inhibit iNOS apart from scavenging peroxynitrite.Citation[15–17],Citation[27] Based on these observations, it is likely that peroxynitrite scavenging may be a therapeutic target of renal I/R injury.
In this work, SMT effectively inhibited an I/R-induced rise in tissue NOx concentration. Measurement of tissue NOx (tissue nitrite/nitrate) provides a reliable and quantitative estimate of NO formation in vivo.Citation[28] NO produced by iNOS during renal I/R contributes to renal injury. The role of NO in the development of renal I/R injury has been confirmed in several investigations where the administration of iNOS inhibitors protect the kidney against I/R injury.Citation[5],Citation[7],Citation[13],Citation[14] In addition, it was shown that the kidney of iNOS knockout mice are resistant to hypoxic injury, whereas endothelial NOS (eNOS) knockout or neuronal NOS (nNOS) knockout were not.Citation[29] On the other hand, it was reported that NO may protect the kidney against I/R injury. L-arginine and NO donors have been shown to improve renal function during I/R.Citation[3],Citation[30],Citation[31] On the basis of the correlation between decreased tissue NOx levels and histological injury scores in our work, it is conceivable that renal I/R injury and ARF originate from excessive production of NO.
It was proposed that cellular damage is attributable to a powerful and cytotoxic oxidant, ONOO—, which is generated by interaction of NO with O2—. Walker et al.Citation[32] found the presence of 3-nitrotyrosine-protein adducts, a marker of peroxynitrite generation, in renal tubules of I/R injured kidneys. In addition, they reported that treatment with the iNOS inhibitor improved renal function and decreased apparent peroxynitrite formation.Citation[32] Peroxynitrite formation has been detected in several investigations of I/R-mediated oxidant injury. Studies have displayed increased direct and/or indirect evidence of peroxynitrite in myocardial, cerebral, and intestinal tissue after I/R injury in vivo.Citation[33–35] It was noted that both MEG and SMT have the same effect on the production of NO. Moreover, the present study revealed that scavenging of peroxynitrite with MEG is more effective in the prevention of injury in the kidney subjected to I/R than inhibition of iNOS by SMT, suggesting that ONOO— may be more important in the pathogenesis of I/R-induced renal injury. Therefore, a pharmacological scavenger of peroxynitrite could be an effective tool for the improvement or prevention of cellular injury in renal I/R.
In conclusion, NO derived from iNOS and ONOO— contribute to the development of cellular injury during renal I/R. This study shows that SMT and MEG reduce the degree of renal dysfunction and injury caused by renal I/R. Scavengers of ONOO— might thus be of therapeutic benefit in conditions associated with renal I/R and ARF.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Rondon-Berrios H, Palevsky PM. Treatment of acute kidney injury: An update on the management of renal replacement therapy. Curr Opin Nephrol Hypertens. 2007; 16: 64–70
- Sheridan AM, Bonventre JV. Pathophysiology of ischemic acute renal failure. Contrib Nephrol. 2001; 7–21
- Chatterjee PK. Novel pharmacological approaches to the treatment of renal ischemia-reperfusion injury: A comprehensive review. Naunyn Schmiedebergs Arch Pharmacol. 2007; 376: 1–43
- Nath KA, Norby SM. Reactive oxygen species and acute renal failure. Am J Med. 2000; 109: 665–678
- Chatterjee PK, Patel NS, Kvale EO, et al. Inhibition of inducible nitric oxide synthase reduces renal ischemia/reperfusion injury. Kidney Int. 2002; 61: 862–871
- Lohinai Z, Benedek P, Feher E, et al. Protective effects of mercaptoethylguanidine, a selective inhibitor of inducible nitric oxide synthase, in ligature-induced periodontitis in the rat. Br J Pharmacol. 1998; 123: 353–360
- Mark LA, Robinson AV, Schulak JA. Inhibition of nitric oxide synthase reduces renal ischemia/reperfusion injury. J Surg Res. 2005; 129: 236–241
- Cerqueira NF, Hussni CA, Yoshida WB. Pathophysiology of mesenteric ischemia/reperfusion: A review. Acta Cir Bras. 2005; 20: 336–343
- Erdogan H, Fadillioglu E, Yagmurca M, Ucar M, Irmak MK. Protein oxidation and lipid peroxidation after renal ischemia-reperfusion injury: Protective effects of erdosteine and N-acetylcysteine. Urol Res. 2006; 34: 41–46
- Harris AG, Leiderer R, Peer F, Messmer K. Skeletal muscle microvascular and tissue injury after varying durations of ischemia. Am J Physiol. 1996; 271: H2388–H2398
- Li C, Jackson RM. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol Cell Physiol. 2002; 282: C227–C241
- Schramm L, Heidbreder E, Schmitt A, et al. Role of L-arginine-derived NO in ischemic acute renal failure in the rat. Ren Fail. 1994; 16: 555–569
- Vinas JL, Sola A, Genesca M, Alfaro V, Pi F, Hotter G. NO and NOS isoforms in the development of apoptosis in renal ischemia/reperfusion. Free Radic Biol Med. 2006; 40: 992–1003
- Noiri E, Nakao A, Uchida K, al et. Oxidative and nitrosative stress in acute renal ischemia. Am J Physiol Renal Physiol. 2001; 281: F948–F957
- Szabo C, Ferrer-Sueta G, Zingarelli B, Southan GJ, Salzman AL, Radi R. Mercaptoethylguanidine and guanidine inhibitors of nitric-oxide synthase react with peroxynitrite and protect against peroxynitrite-induced oxidative damage. J Biol Chem. 1997; 272: 9030–9036
- Szabo A, Hake P, Salzman AL, Szabo C. Beneficial effects of mercaptoethylguanidine, an inhibitor of the inducible isoform of nitric oxide synthase and a scavenger of peroxynitrite, in a porcine model of delayed hemorrhagic shock. Crit Care Med. 1999; 27: 1343–1350
- Zingarelli B, Cuzzocrea S, Szabo C, Salzman AL. Mercaptoethylguanidine, a combined inhibitor of nitric oxide synthase and peroxynitrite scavenger, reduces trinitrobenzene sulfonic acid-induced colonic damage in rats. J Pharmacol Exp Ther. 1998; 287: 1048–1055
- Szabo C, Southan GJ, Thiemermann C. Beneficial effects and improved survival in rodent models of septic shock with S-methylisothiourea sulfate, a potent and selective inhibitor of inducible nitric oxide synthase. Proc Natl Acad Sci USA. 1994; 91: 12472–12476
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193: 265–275
- Levine RL, Garland D, Oliver CN, et al. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990; 186: 464–478
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95: 351–358
- Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988; 34: 497–500
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967; 70: 158–169
- Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001; 5: 62–71
- Patel NS, Chatterjee PK, Chatterjee BE, et al. TEMPONE reduces renal dysfunction and injury mediated by oxidative stress of the rat kidney. Free Radic Biol Med. 2002; 33: 1575–1589
- Guz G, Demirogullari B, Ulusu NN, et al. Stobadine protects rat kidney against ischaemia/reperfusion injury. Clin Exp Pharmacol Physiol. 2007; 34: 210–216
- Zingarelli B, Southan GJ, Gilad E, O'Connor M, Salzman AL, Szabo C. The inhibitory effects of mercaptoalkylguanidines on cyclo-oxygenase activity. Br J Pharmacol. 1997; 120: 357–366
- Grzelec-Mojzesowicz M, Sadowski J. Renal tissue NO and intrarenal hemodynamics during experimental variations of NO content in anesthetized rats. J Physiol Pharmacol. 2007; 58: 149–163
- Ling H, Gengaro PE, Edelstein CL, et al. Effect of hypoxia on proximal tubules isolated from nitric oxide synthase knockout mice. Kidney Int. 1998; 53: 1642–1646
- Kucuk HF, Kaptanoglu L, Ozalp F, et al. Role of glyceryl trinitrate, a nitric oxide donor, in the renal ischemia-reperfusion injury of rats. Eur Surg Res. 2006; 38: 431–437
- Jeong GY, Chung KY, Lee WJ, Kim YS, Sung SH. The effect of a nitric oxide donor on endogenous endothelin-1 expression in renal ischemia/reperfusion injury. Transplant Proc. 2004; 36: 1943–1945
- Walker LM, Walker PD, ImamS Z, Ali SF, Mayeux PR. Evidence for peroxynitrite formation in renal ischemia-reperfusion injury: Studies with the inducible nitric oxide synthase inhibitor L-N(6)-(1-Iminoethyl)lysine. J Pharmacol Exp Ther. 2000; 295: 417–422
- Sharma SS, Munusamy S, Thiyagarajan M, Kaul CL. Neuroprotective effect of peroxynitrite decomposition catalyst and poly(adenosine diphosphate-ribose) polymerase inhibitor alone and in combination in rats with focal cerebral ischemia. J Neurosurg. 2004; 101: 669–675
- Liu P, Hock CE, Nagele R, Wong PY. Formation of nitric oxide, superoxide, and peroxynitrite in myocardial ischemia-reperfusion injury in rats. Am J Physiol. 1997; 272: H2327–H2336
- Cuzzocrea S, Misko TP, Costantino G, et al. Beneficial effects of peroxynitrite decomposition catalyst in a rat model of splanchnic artery occlusion and reperfusion. FASEB J. 2000; 14: 1061–1072