Abstract
Objectives. To investigate the correlation between concentrations of asymmetric dimethylarginine (ADMA) and neopterin (NP) as potential risk factors for cardiovascular diseases in chronic renal failure patients. Method. In this study, 33 patients with renal failure before and after haemodialysis were compared with healthy control subjects. Serum ADMA and NP levels were measured using high performance liquid chromatography (HPLC). Results. When ADMA and NP concentrations in renal failure patients were compared before and after dialysis, before dialysis ADMA and NP concentrations were higher than those in the control group. However, ADMA and NP levels showed a falling mean and clear after dialysis. While there is no correlation between ADMA and NP levels before dialysis, there is a mean and positive correlation between ADMA and NP levels after dialysis. Conclusion. Potential risk factors for cardiovascular diseases include high concentrations of both ADMA and NP levels in chronic renal failure patients. A correlation mean between ADMA and NP levels after dialysis was found, but no correlation between ADMA and NP levels before haemodialysis was discovered. These can be evaluated as two different risk factors independent from each other.
INTRODUCTION
Atherosclerotic vascular complications are the major cause of morbidity and mortality in patients with different stages of chronic renal disease.Citation[1] In recent years, there has been a growing body of evidence that even minor renal dysfunction is associated with high risks of cardiovascular events. Now, chronic kidney disease (CKD) is generally thought to be one of the risk factors for cardiovascular disease (CVD). Because endothelial dysfunction is an initial step to atherosclerosis in patients with hypertension, diabetes, and CKD, reduced generation and/or bioavailability of nitric oxide (NO) may link these risk factors to the events of CVD.Citation[2] The association between conventional risk factors, together with malnutrition and chronic inflammation, seems to be a major source of oxidative stress, playing an important role in the pathogenesis of dependent-NO endothelial dysfunction.Citation[3]
Nitric oxide (NO) is very active but short living, and has harmful effects on cell activity. NO is a critical modulator of blood flow and blood pressure. It is released by the endothelium in response to shear stress and plays an important role in flow-mediated vasodilatation.Citation[4] The diminished bioavailability of NO impairs endothelium-dependent vasodilatation and activates other mechanisms that may play an important role in the pathogenesis of atherosclerosis.Citation[5] NO, synthesized from L-arginine, accounts for the powerful vasodilator effects of endothelium-derived relaxing factor and consequently plays a decisive role in determining vasomotor tone.Citation[6] Asymmetric dimethylarginine (ADMA) is an endogenous competitive inhibitor of NO synthase that can modulate NO production.Citation[7–9] It is synthesized and metabolized by human endothelial cells.Citation[10] ADMA and its regioisomer, symmetric dimethylarginine (SDMA), are generated by the degradation of methylated proteins due to protein arginine methyltransferase 1 (PRMT 1) activityCitation[11] and subsequent physiological protein turnover.Citation[8] ADMA is eliminated from the body either by renal excretion or by degradation to dimethylamine and citrulline by the enzyme dimethylarginine dimethylaminohydrolase (DDAH).Citation[12] ADMA, but not SDMA, is an endogenous inhibitor of endothelial nitric oxide synthase (eNOS).Citation[8] ADMA inhibits eNOS by competitive displacement of the physiological substrate, L-arginine, from the enzyme.Citation[13],Citation[14] The inhibition leads to decrease in NO production in the endothelium of vessel walls. Thus, ADMA levels are elevated, and endothelial dysfunction may result.Citation[14] Further, ADMA level was recently found to be elevated in patients with CKD and atherosclerotic vascular complications.Citation[15],Citation[16] In addition, plasma ADMA level predicts future cardiovascular events and the progression of renal injury in patients with CKD.Citation[17],Citation[18]
Recently, the potential role of ADMA in CKD was comprehensively reviewed by Zoccali et al. (2006).Citation[19] According to their theory, there are at least four possible mechanisms that may explain the accumulation of ADMA in CKD:
increased methylation of proteins;
increased protein turnover;
decreased metabolism by DDAH; and
impaired renal excretion.
As dimethylarginines are excreted in urine,Citation[20] impaired renal clearance may, at least in part, account for the elevation of ADMA levels in patients with CKD.
Neopterin (NP), belonging to the pteridine family, is synthesized primarily by human monocytes and macrophages after stimulation by interferon-gamma (IFN-γ) produced by activated helper T-cells.Citation[21],Citation[22] The biosynthesis of neopterin starts from purinic guanosine triphosphate base that is converted to 7,8-dihydroneopterintriphosphate by GTP ciclohydrolase I.Citation[23] NP levels rise in many pathologic conditions: renal failureCitation[24]; diabetic nephropathyCitation[25]; glomerulonephritisCitation[26],Citation[27]; renal transplant rejectionCitation[28]; insulin resistanceCitation[29]; autoimmune diseasesCitation[30]; viral,Citation[31] bacterial,Citation[32] and protozoic infectionsCitation[33]; coronary artery disease Citation[34]; and psoriasis.Citation[35] In humans, there is a correlation between serum NP concentration and the extent of atherosclerosis.Citation[36] In patients undergoing haemodialysis, serum NP levels decrease as a consequence of the procedure.Citation[37] In renal diseases, two mechanisms are responsible for increased NP concentrations: impaired excretion of the compound and its production in the course of inflammation.
Studies have reported an association between ADMA and NP levels and the grade of atherosclerosis.Citation[38] These findings suggest that the elevation of ADMA and NP may be a missing link between CVD and CKD. The purpose of the present study was to investigate any relationship between concentrations of asymmetric dimethylarginine and neopterin as potential risk factors for cardiovascular diseases in chronic renal failure patients, with control subject.
METHODS
Patients
Thirty-three patients with haemodialysis treatment at Gazi University, Faculty of Medicine, (Ankara, Turkey) were examined in this study. Dialyzer membrane type was polysulfone semisynthetic and dialyzer machine was Fresenius 4008B. We studied three groups of individuals: 33 healthy subjects, 33 subjects with renal failure disease that were being prepared before dialysis, and 33 subjects with renal failure disease after dialysis. Then, the samples were stored at −80°C.
Biochemical Analysis
Measurement of ADMA by HPLC Methods
Measurement of ADMA was accomplished by HPLC using a method described by Chen et al. Citation[39] In brief, 20 mg 5-sulfosalisilic acid was added to 1 ml serum, and the mixture was left in an ice bath for 10 min. The precipitated protein was removed by centrifugation at 2000 × g for 10 min. Ten microlitres of the supernatant, which was filtered through a 0.2 μm-pore size filter, was mixed with 100 μl derivatization reagent (prepared by dissolving 10 mg ο-phtaldialdehyde in 0.5 ml methanol, 2 ml 0.4 M borate buffer (pH10.0), and 30 μl 2-mercartoethanol were added) and then injected into the chromatographic system. Separation of ADMA was achieved with a 150 × 4 mm-interior diameter Nova-Pak C18 column with a particle size of 5 μm (Waters, Millipore Corp., Milford, Massachusetts, USA) using 50 mM sodium acetate (pH 6.8), methanol, and tetrahydrofurane as mobile phase (A; 82/17/1, B; 22/77/1 [v/v/v], respectively) at a flow rate of 1.0 ml/min. The areas of peaks detected by fluorescent detector (excitations, 338 nm; emission, 425 nm) were used for quantification. The intra- and inter-assay coefficients of variation for ADMA were 2.8% and 4.5%, respectively.
Measurement of NP by HPLC Methods
The measurement of NP was accomplishment by HPLC. In this study, measurements were made using a C18/5 μm reverse-phase column 4.6 × 250 mm, and the most appropriate mobile phase has been determinate to be 0.015 mol/L phosphate buffer with pH 6.4 as a consequence of various optimization studies. For the neopterin analysis, 100 μL of TCA (2 mol/L) were added to 100 μL of standard or serum sample and vortexed for 10 s. The samples were centrifuged at 10,000 g for 10 min. Then, 50 μL of supernatant was diluted with 200 μL of bidistilled water, and 20 μL aliquot was used for HPLC analysis. The areas of peaks detected by fluorescent detector (excitations, 353 nm; emission, 438 nm) were used for quantification. The intra- and inter-assay coefficients of variation for NP were 4.7% and 6.9%.
Statistical Analysis
All data were expressed in terms of mean ± standard deviation. Statistics were done using the software Statistical Package of Social Science (SPSS 11.0.0, 2001; SPSS Inc., Chicago, Illinois, USA). The Mann-Whitney U-test was used to compare differences between two independent groups, and the Spearman rank correlation test was used for estimating relationships between variables. A p value of ≤0.05 was considered statistically significant.
RESULTS
The characteristics of patients; BUN, creatinine, ADMA, and NP levels; and other parameters both before dialysis and after dialysis in patients with chronic renal failure and control subjects are shown in . ADMA and NP levels were not affected by age, sex, HD vintage, original CKD, or HD membrane in our study group.
Table 1 Characteristics of patients. Comparison of ADMA and Neopterin levels between before dialysis and after dialysis in patients with chronic renal failure and control subjects
Serum ADMA Concentrations in Renal Failure
The serum concentrations of ADMA are low in healthy subjects (ADMA: 0.89 ± 0.26 μmol/L). This study was compared with control subjects; HD-treated patients exhibited significantly (p < 0.05) higher serum ADMA concentrations. However, serum ADMA concentrations were determined before dialysis and after dialysis in patients with chronic renal failure. In our results, ADMA levels were found to be significantly (p < 0.05) higher before dialysis (3.14 ± 2.50 μmol/L) than after dialysis (1.53 ± 1.18 μmol/L). Nevertheless, high ADMA levels are not enough to eliminate by dialysis.
Serum NP Concentrations in Renal Failure
The serum concentrations of NP are very low in healthy subjects (NP: 19 ± 7 nmol/L). In our study, serum NP concentrations were measured both before dialysis and after dialysis in patients with chronic renal failure. Serum NP concentrations were greater before dialysis (291 ± 135 nmol/L) than after dialysis (182 ± 98 nmol/L). Nevertheless, this reduction does not reach normal serum NP concentration levels. Also, compared with control subjects, HD-treated patients exhibited significantly (p < 0.05) higher serum NP concentrations.
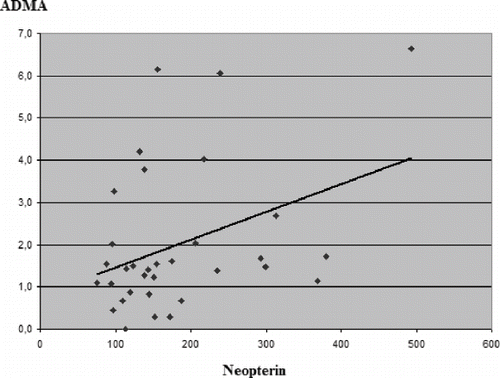
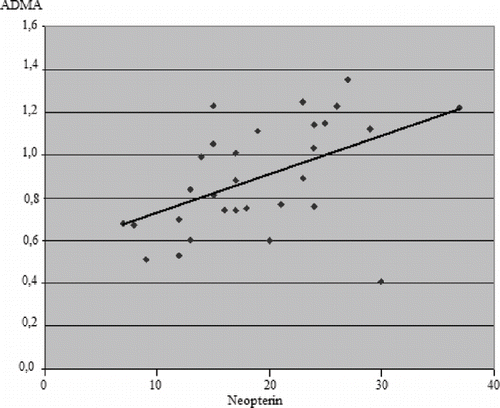
We also analyzed any association between levels of ADMA, NP, and uremic molecules. The NP levels correlated positively with ADMA levels in both HD-treated patients and control groups (r = 0.381, p < 0.05; r = 0.497; p < 0.05, respectively; see and ). There were no correlations between uremic molecules and ADMA or NP.
DISCUSSION
Recently, biochemical factors including ADMA and NP showed in most diseases, such as renal failure, diabetic disease, cardiovascular disease, and hepatitis.Citation[40] Several investigators have proposed ADMA as a potentially useful biomarker in cardiovascular disease. Until recently, there was no compelling evidence that ADMA plays a causal role in the pathophysiology of vascular disease. Thus, although elevated ADMA levels have been frequently associated with various cardiovascular risk factors, it remained unclear as to whether this molecule is simply a marker or also a “maker” of vascular disease.Citation[41] Despite the wide range of reported ADMA levels in patients with renal disease, there is some evidence that ADMA may be a useful marker to assess severity of renal disease. Most studies are of limited value, as they were cross-sectional in nature and simply show an association of elevated ADMA levels with renal disease.Citation[42],Citation[43]
Our finding that ADMA levels are significantly higher in patients with chronic renal failure than in healthy control subjects is consistent with the first description by Vallance and colleaguesCitation[8] of nine HD-treated patients. They reported a mean total ADMA level of 8.7 ± 0.7 μmol/L. This concentration was six times higher than that measured for control subjects (1.2 ± 0.1 μmol/L).Citation[8] In our study, ADMA level before dialysis in patients was found to be three times higher than in healthy subjects, a statistically significant finding (p < 0.05). However, ADMA level after dialysis was found to be lower than before dialysis. MacAllister and colleagues found elevated ADMA level (0.9 ± 0.1 μmol/L) in six HD-treated patients.Citation[12] Kielstein and colleagues observed that the plasma concentration of ADMA was higher in patients with end-stage renal disease and atherosclerotic vascular disease than in those without vascular complications.Citation[44] Miyazaki and colleagues measured dimethylarginine levels in the serum of 116 human subjects who had no signs of coronary or peripheral arterial disease, and found that ADMA levels were positively correlated with age, mean arterial pressure, and glucose tolerance. Most intriguingly, ADMA levels were significantly correlated with carotid artery intima-media thickness in a stepwise regression analysis of this population.Citation[45] As the relationship between carotid intima-media thickness and major cardiovascular events had also recently been established,Citation[46],Citation[47] these authors proposed that ADMA is a marker of cardiovascular disease.
Irrespective of its absolute concentration, serum ADMA levels correlate significantly with established risk factors of atherosclerosis in both renal and nonrenal patients.Citation[15],Citation[44],Citation[45],Citation[47–51] Thus, ADMA is thought to be not only a novel biochemical marker of atherosclerosis, but it may even be causally involved in the pathogenesis of atherosclerotic disease.Citation[4]
Neopterin, which is a biochemical marker of cellular immune response, belongs to the group of compounds known as pteridines. The first pteridines were initially identified in 1889, and in 1979, Wachter et al. reported that urine neopterin levels were elevated in cases of viral infections and tumors.Citation[53] Recently, NP has been reported as the indicator of local macrophage activity in different body fluids.Citation[54] Neopterin is biologically stable, and increased concentrations in serum and urine have been used as a diagnostic and prognostic criterion for cell-mediated immunity in some clinical conditions. Because neopterin excretion takes place before clinical symptoms appear, biochemical follow-up of neopterin levels has been accepted as a strong indicator for the clinical severity of some diseases.Citation[55] Further, a marked abnormality in pteridine metabolism has been noted in cases of chronic renal disease.Citation[56] Pecoits-Filho et al. also reported that neopterin levels were significantly higher in the subgroup of patients with a low glomerular filtration rate, suggesting either impaired renal elimination, increased generation in uremia, or an adverse effect of inflammation on renal function.Citation[57] In our study, there was a trend toward increased serum neopterin levels in patients with chronic renal failure compared to the control group that was statistically significant (p < 0.05).
A possible explanation for the prognostic value of neopterin, as observed separately by GuptaCitation[58] and Schumacher,Citation[59] is that neopterin is a marker of coronary disease activity. Neopterin affects intracellular redox state and has been shown to be involved in the activation of both constitutive and inducible NO synthase.Citation[60],Citation[61]
Previously, neopterin has been used as a marker of the stimulation of the cellular immune system. Our finding that neopterin is higher in patients with chronic renal failure than in healthy control subjects is consistent with previous reports, which have shown that coronary syndromes are associated inflammatory mechanisms.Citation[62],Citation[63]
CONCLUSION
Our study researched the relationship between ADMA and NP concentrations as potential risks factors for cardiovascular disease in patients with chronic renal failure. We have found elevated ADMA and NP levels in patients with chronic renal failure compared with controls. There found a correlation mean between ADMA and NP levels after dialysis, but there was not found any correlation between ADMA and NP levels before haemodialysis. However, a correlation between ADMA and NP levels after haemodilaysis may be evaluated in terms of dialysis effect. These can be evaluated as two different risk factors independent from each other. In addition, in examining this decrease, it is reasonable to assume that hemodialysis is not more effective with respect to ADMA and NP elimination than the kidney.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Parfrey PS, Foley RN. The clinical epidemiology of cardiac disease in chronic renal failure. J Am Soc Nephrol. 1999; 10: 1606–1615
- Ueda S, Yamagihi S, Kaida Y, Okuda S. Asymmetric dimethylarginine may be a missing link between cardiovascular disease and chronic kidney disease. Nephrology. 2007; 12: 582–590
- Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM. The elephant in uremia: Oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002; 62: 1524–1538
- Cooke JP. Does ADMA cause endothelial dysfunction?. Arterioscler Thromb Vasc Biol. 2000; 20: 2032–2037
- Ross R. The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature. 1993; 362: 801–809
- Cakir E, Ozcan O, Yaman H, Akgul EO, Bilgi C, Erbil ME, Yesilova Z. Elevated plasma concentration of asymmetric dimethylarginine that is reduced by single dose testosterone administration in idiopathic hypogonadotropic hypogonadism patients. Journal of Clinical Endocrinology & Metabolism. 2004; 90(3)1651–1654
- Leiper J, Vallance P. Biological significance of endogenous methylargines that inhibit nitric oxide synthase. Cardiovasc Res. 1999; 43: 542–548
- Vallance P, Leone A, Calver A, Collier J, Moncada S. Endogenous dimethylarginine as an inhibitor of nitric oxide synthesis. J Cardiovasc Pharmacol. 1992; 20(Suppl. 12)60–62
- Bozlar U, Ugurel MS, Ozcan O, Cakir E, Ustunsoz B, Ucoz T, Bilgi C, Somuncu I. Impact of catheter arteriography on the serum level of asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase. Cardiovasc Intervent Radiol. May–Jun, 2008; 31(3)490–495
- MacAllister RJ, Fickling SA, Whitley GS, Vallance P. Metabolism of methylarginines by human vasculature: Implications for the regulation of nitric oxide synthesis. Br J Pharmacol. 1994; 112: 43–48
- McDermott JR. Studies on the catabolism of Ng-methylarginine, N9g, Ng-dimethylarginine and Ng, Ng-dimethylarginine in the rabbit. Biochem J. 1976; 154: 179–184
- MacAllister RJ, Parry H, Kimoto M, Ogawa T, Russell RJ, Hodson H, et al. Regulation of nitric oxide synthesis by dimethylarginine dimethylaminohydrolase. Br J Pharmacol. 1996; 119: 1533–1540
- Albsmeier J, Tsikas D, Frolich JC, Boger RH. Asymmetric dimethylarginine (ADMA), an explanation for the L-arginine paradox. Naunyn-Schmiedeberg Arch Pharmacol. 2002; 365(Suppl)R14–R44
- Boger RH, Bode-Boger SM, Szuba A, Tsao PS, Chan JR, Tangphao O, et al. Asymmetric dimethylarginine (ADMA): A novel risk factor for endothelial dysfunction: Its role in hypercholesterolemia. Circulation. 1998; 98: 1842–1847
- Zoccali C, Benedetto FA, Maas R, et al. Asymmetric dimethylarginine, C-reactive protein, and carotid intima-media thickness in end-stage renal disease. J Am Soc Nephrol. 2002; 13: 490–496
- Cooke JB. Asymmetrical dimethylarginine: The Uber marker?. Circulation. 2004; 109: 1813–1818
- Fliser D, Kronenberg F, Kielstein JT, et al. Asymmetric dimethylarginine and progression of chronic kidney disease: The mild to moderate kidney disease study. J Am Soc Nephrol. 2005; 16: 2456–2461
- Ravani P, Tripepi G, Malberti F, Testa S, Mallamaci F, Zoccali C. Asymmetrical dimethylarginine predicts progression to dialysis and death in patients with chronic kidney disease: A competing risks modeling approach. J Am Soc Nephrol. 2005; 16: 2449–2455
- Zoccali C. Asymmetric dimethylarginine in end-stage renal disease patients: A biomarker modifiable by calcium blockade and angiotensin II antagonism. Kidney Int. 2006; 70: 2053–2055
- Kakimoto Y, Akazawa S. Isolation and identification of NG, NG- and NG, N'G-dimethyl-arginine, N-mono-, di-, and trimethyllysine, and glucosylgalactosyl-, and galactosyl-d-hydroxylysine from human urine. J Biol Chem. 1970; 245: 5751–5758
- Huber C, Batchelor JR, Fuchs D, Hausen A, Lang A, Niederwieser D, Reibnegger G, Swetly P, Troppmair J, Wachter H. Immune response-associated production of neopterin. Release from macrophages primarily under control of interferon gamma. Journal of Experimental Medicine 1984; 160: 310–316
- Celada A, Gray PW, Rinderknecht E, Schreiber RD. Evidence for a gamma-interferon receptor that regulates macrophage tumoricidal activity. Journal of Experimental Medicine. 1984; 160: 55–74
- Duch DS, Bowers SW, Woolf JH, Nichol CA. Biopterin cofactor biosynthesis: GTP cyclohydrolase, neopterin and biopterin in tissues and body fluids of mammalian species. Life Sci. 1984; 35: 1895–1901
- Roccatello D, Formica M, Cavalli G, et al. Serum and intracellular detection of cytokines in patients undergoing chronic hemodialysis. Artificial Organs. 1992; 16: 131–140
- Weiss MF, Rodby RA, Justice AC, Hricik DE. Free pentosidine and neopterin as markers of progression rate in diabetic nephropathy. Kidney International. 1998; 54: 193–202
- Oda K, Arai T, Nagase M. Increased serum and urinary neopterin in nephrotic syndrome indicate cell-mediated immune dysfunction. Am J Kidney Dis. 1999; 34: 611–617
- Godai K, Uemasu J, Kawasaki H. Clinical significance of serum and urinary neopterins in patients with chronic renal disease. Clinical Nephrology. 1991; 36: 141–146
- Kotanko P, Margreiter R, Pfaller W. Urinary N-acetyl-beta-D-glucosaminidase and neopterin aid in the diagnosis of rejection and acute tubular necrosis in initially nonfunctioning kidney grafts. Nephron. 2000; 84: 228–235
- Ledochowski M, Murr C, Widner B, Fuchs D. Association between insulin resistance, body mass and neopterin concentrations. Clinical Chimica Acta. 1999; 282: 115–123
- Widner B, Sepp N, Kowald E, Kind S, Schmuth M, Fuchs D. Degradation of tryptophan in patients with systemic lupus erythematosus. Adv ExpMedical Biology. 1999; 467: 571–577
- Baier-Bitterlich G, Fuchs D, Wachter H. Chronic immune stimulation, oxidative stress, and apoptosis in HIV infection. Biochem Pharmacol. 1997; 53: 755–763
- Fuchs D, Hausen A, Kofler M, Kosanowski H, Reibnegger G, Wachter H. Neopterin as an index of immune response in patients with tuberculosis. Lung. 1984; 162: 337–346
- Reibnegger G, Boonpucknavig V, Fuchs D, Hausen A, Schmutzhard E, Wachter H. Urinary neopterin is elevated in patients with malaria. Trans. Royal Society of Tropical Medicine and Hygiene 1984; 78: 545–546
- Garcia-Moll X, Cole D, Zouridakis E, Kaski JC. Increased serum neopterin: A marker of coronary artery disease activity in women. Heart. 2000; 83: 346–350
- Sanchez-Regana M, Catasus M, Creus L, Umbert P. Serum neopterin as an objective marker of psoriatic disease activity. Acta Dermatological Venereologica 2000; 80: 185–187
- Weiss G, Willeit J, Kiechl S, et al. Increased concentrations of neopterin in carotid atherosclerosis. Atherosclerosis. 1994; 106: 263–271
- Roccatello D, Formica M, Cavalli G, et al. Serum and intracellular detection of cytokines in patients undergoing chronic hemodialysis. Artificial Organs. 1992; 16: 131–140
- Tatzber F, Rabl H, Korisk K, Erhart U, Puhl H, Waeg G, et al. Elevated serum neopterin levels in atherosclerosis. Atherosclerosis. 1991; 89: 203–208
- Chen BM, Xia LW, Zhao RQ. Determination of NG, NG -dimethylarginine in human plasma by high-performance liquid chromatography. J Chromatography B. 1997; 2: 467–471
- Ozcan O, Cakir E, Yaman H, Akgul EO, Erturk K, Beyhan Z, Bilgi C, Erbil MK. The effects of thyroxine replacement on the levels of serum asymmetric dimethylarginine (ADMA) and other biochemical cardiovascular risk markers in patients with subclinical hypothyroidism. Clin Endocrin. 2005; 63: 203–206
- Leiper J, Nandi M, Torondel B, Murray-Rust J, Malaki M, O'Hara B, Rossiter S, Anthony S, Madhani M, Selwood D, Smith C, Wojciak-Stothard B, Rudiger A, Stidwill R, McDonald NQ, Vallance P. Disruption of methylarginine metabolism impairs vascular homeostasis. Nat Med. 2007; 13: 198–203
- Ravani P, Tripepi G, Malberti F, Testa S, Mallamaci F, Zoccali C. Asymmetrical dimethylarginine predicts progression to dialysis and death in patients with chronic kidney disease: A competing risks modeling approach. J Am Soc Nephrol. 2005; 16: 2449–2455
- Yilmaz MI, Saglam M, Caglar K, Cakir E, Sonmez A, Ozgurtas T, Aydin A, Eyileten T, Ozcan O, Acikel C, Tasar M, Genctoy G, Erbil K, Vural A, Zoccali C. The determinants of endothelial dysfunction in CKD: Oxidative stress and asymmetric dimethylarginine. Am J Kidney Dis. Jan, 2006; 47(1)42–50
- Kielstein JT, Böger RH, Bode-Böger SM, Schaffer J, Barbey M, Koch KM, Frölich JC. Asymmetric dimethylarginine plasma concentrations differ in patients with end-stage renal disease: Relationship to treatment method and atherosclerotic disease. J Am Soc Nephrol. 1999; 10: 594–600
- Miyazaki H, Matsuoka H, Cooke JP. Endogenous nitric oxide synthase inhibitor. A novel marker of atherosclerosis. Circulation. 1997; 80: 331–333
- Hodis HN, Mack WJ, LaBree L, et al. The role of carotid arterial peripheral arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998; 128: 262–269
- O'Leary DH, Polak JF, Kronmal RA, et al. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Project. New Engl J Med. 1999; 340: 14–22
- Fard A, Tuck CH, Donis JA, Sciacca R, Di Tullio MR, Wu HD, Bryant TA, Chen NT, Torres-Tamayo M, Ramasamy R, Berglund L, Ginsberg HN, Homma S, Cannon PJ. Acute elevations of plasma asymmetric dimethylarginine and impaired endothelial function in response to a high-fat meal in patients with type 2 diabetes. Arterioscler Thromb Vasc Biol. 2000; 20: 2039–2044
- Yilmaz MI, Saglam M, Sonmez A, Caglar K, Cakir E, Kurt Y, Eyileten T, Tasar M, Acikel C, Oguz Y, Vural A, Yenicesu M. Improving proteinuria, endothelial functions and asymmetric dimethylarginine levels in chronic kidney disease: Ramipril versus valsartan. Blood Purif. 2007; 25(4)327–335
- Kielstein JT, Böger RH, Bode-Böger SM, Frölich JC, Haller H, Ritz E, Fliser D. Marked increase of asymmetric dimethylarginine in patients with incipient primary chronic renal disease. J Am Soc Nephrol. 2002; 13: 170–176
- Yoo JH, Lee SC. Elevated levels of plasma homocyst(e)ine and asymmetric dimethylarginine in elderly patients with stroke. Atherosclerosis. 2001; 158: 425–430
- Zoccali C, Bode-Böger S, Mallamaci F, Benedetto F, Tripepi G, Malatino L, Cataliotti A, Bellanuova I, Fermo I, Frölich J, Böger R. Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: A prospective study. Lancet. 2001; 358: 2113–2117
- Wachter H, Hausen A, Grassmayr K. Increased urinary excretion of neopterin in patients with malignant tumors and with virus diseases. Hoppeseyler's Z Physiol Chem. 1979; 360: 1957–1960
- Muller MM, Curtius HC, Herold M, Huber CH. Neopterin in clinical practica. Clin Chem Acta. 1991; 201: 1–16
- Yaman H, Cakir E, Ozcan O, Yesilova Z, Ozcan A, Akgul EO, Erbil MK, Bagci S, Bilgi C, Dagalp K. Elevated urine neopterin levels in nonalcoholic steatohepatitis. Clin Biochem. 2005; 38: 187–190
- Yokoyama K, Tajima M, Yoshida H, Nakayama M, Tokutome G, Sakagami H, Hosoya T. Plasma pteridine concentrations in patients with chronic renal failure. Nephrol Dial Transplant. 2002; 17: 1032–1036
- Pecoits-Filho R, Heimburger O, Barany P, Suliman M, Fehrman-Ekholm I, Lindholm B, Stenvinkel P. Associations between circulating inflammatory markers and residual renal function in CRF patients. Am J Kidney Dis. 2003; 41: 1212–1218
- Gupta S, Fredericks S, Schwartzman RA, et al. Serum neopterin in acute coronary syndromes. Lancet. 1997; 349: 1252–1253
- Schumacher M, Halwachs G, Tatzber F, et al. Increased neopterin in patients with chronic and acute coronary syndromes. J Am Coll Cardiol. 1997; 30: 703–707
- Schobersberger W, Hoffmann G, Grote J, et al. Induction of inducible nitric oxide synthase expression by neopterin in vascular smooth muscle cells. FEBS Lett. 1995; 377: 461–464
- Werner-Felmayer G, Werner ER, Fuchs D, et al. Pteridine biosynthesis in human endothelial cells. Impact on nitric oxide-mediated formation of cyclic GMP. J Biol Chem. 1993; 268: 1842–1846
- Neri Serneri GG, Prisco D, Martini F, et al. Acute T-cell activation is detectable in unstable angina. Circulation. 1997; 95: 1806–1812
- De Servi S, Mazzone A, Ricevuti G, et al. Clinical and angiographic correlates of leukocyte activation in unstable angina. J Am Coll Cardiol. 1995; 26: 1146–1150