Abstract
Oxidative stress is a key cause in the development of diabetic nephropathy (DN). As a main receptor of oxidized low-density lipoprotein (oxLDL), LOX-1 plays an important role in the induction of leukocyte adhesion molecules, such as intercellular cell adhesion molecule-1 (ICAM-1). Taurine (TAU), a potent endogenous antioxidant, showed renoprotective effects in several model animals. This study was designed to determine the renoprotective effect and possible mechanism involved LOX-1 and ICAM-1 expression of taurine in early DN. Six-week-old male Wistar rats were divided into three groups: normal control (NC), diabetes mellitus (DM), and taurine-treated DM (DM+TAU). Diabetes was induced by streptozotocin (STZ, 60 mg/kg, i.p.). After the onset of diabetes, drinking water containing 1% taurine was given to rats in the DM+TAU group. After six weeks of treatment, blood glucose (BG), serum levels of creatinine (sCr) and BUN, and LOX-1 and ICAM-1 expression (protein and gene) in kidney cortices were estimated. Meanwhile, renal malondialdehyde (MDA) levels and glutathione peroxidase (GSH-Px) activities were examined as parameters of oxidative stress in diabetic rats. For DM+TAU rats, when compared with DM rats, the levels of serum BUN, sCr, and renal MDA were reduced, and the activities of renal GSH-Px were increased, but the BG levels were not influenced. Simultaneously, taurine attenuated histopathologic evidence of renal damages and reduced the overexpression of LOX-1 and ICAM-1 in kidney cortices of diabetic rats. In conclusion, taurine showed protective effects against early renal injury in diabetic rats. These renoprotective effects may be partly caused by suppression of oxLDL/LOX-1 system and subsequently ICAM-1 overexpression on renal cortex via its antioxidative property.
INTRODUCTION
Diabetic nephropathy (DN) is one major microangiopathy of diabetes mellitus, often leading to end-stage renal failure, in which oxidative stress induced by hyperglycemia is considered to play a critical role.Citation[1],Citation[2] Oxidized LDL, as a product of the exposure of LDL to oxidative stress, plays an important role in the progression of DN.Citation[3] The lectin-like oxLDL receptor-1, LOX-1, is a mainly endothelial receptor that mediates the internalization and degradation of oxLDL by endothelial cells.Citation[4] The expression of LOX-1 is upregulated by oxidative stress, and more importantly, through oxLDL binding to LOX-1, oxLDL further induces the generation of ROS and activates the redox-sensitive transcription nuclear factor-kB (NF-kB),Citation[5] which may further induce intercellular adhesion molecule-1 (ICAM-1) expression in endothelial and mesangial cell.Citation[6] It is suggested that the overexpression of ICAM-1 on glomerular endothelial cells may induce the recruitment of macrophages into glomeruli and plays a critical role on renal injury induced by diabetes in the early stage.Citation[7],Citation[8] Recent studies have showed that LOX-1 expression was highly upregulated in the kidneys of experimental hypertension, ischemia-reperfusion, and hypercholesterolemia,Citation[9–11] as well as of chronic renal failure models induced by 5/6 nephrectomizing.Citation[12] Thus, it is plausible to speculate that LOX-1-mediated ICAM-1 overexpression may enhance the microvascular dysfunction caused by oxLDL, leading to the development and progression of DN.
Tight glycemia control delays the onset and progression of microvascular complications in diabetic patients and animals.Citation[13],Citation[14] However, tight glycemia control in diabetes is difficult and sometimes dangerous. Attention has been developed to the beneficial biochemical function of natural or endogenous antioxidants for the prevention or protection against organ damage caused by diabetes.Citation[15],Citation[16] Taurine, an important intracellular free beta-amino acid, has been shown to be a potent endogenous antioxidant that protects cellular membranes against toxic compounds by inhibition lipid peroxidation and neutrophil activation.Citation[17] Recent studies suggested that taurine could protect against renal damages induced by different models via decreasing production of oxidative stress and enhancing activities of antioxidant enzymes.Citation[18],Citation[19] Research proved that taurine had protective or preventive effects on many diabetic microangiopathy, such as diabetic cardiomyopathy and polyneuropathy, via different mechanisms.Citation[20],Citation[21] But there are still very few published reports focused on the effects and possible mechanisms of taurine on early DN.
Therefore, in the present study, we investigated the effects of taurine on early DN in STZ-induced rats and the possible mechanism involved the role of LOX-1 by examining LOX-1 and ICAM-1 expression on kidney cortex as well as parameters of oxidative stress in early stage of diabetes.
MATERIALS AND METHODS
Animal Model
Six-week-old male Wistar rats (160–180g) were obtained from the Experimental Animal Center of Shandong University. They received humane care in compliance with the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH publication 86–23, revised 1986). Diabetes was induced by a single intraperitoneal injection of STZ (60 mg/kg, Sigma, USA). The normal control rats (NC group, n = 8) were injected with an equal volume of vehicle. After 72 hours following STZ injection, animals showing blood glucose higher than 16.7 mmol/L were considered as diabetic rats. Then, randomly divided diabetic rats into two groups: the diabetes mellitus group (DM group, n = 8) and the taurine-treated group (DM + TAU group, n = 8; drinking water containing 1% taurine [TAU] from Alphar Aesar, USA). All rats were maintained on a 12h light/dark cycle and kept for six weeks with free access to food and water. All protocols were in accordance with the institutional guidelines for animal research.
Metabolic Parameters and Tissue Collection
At the end of six weeks, after fasting overnight, all rats were anesthetized with 3% butylene (50 mg/kg, i.p.) and sacrificed. Blood samples were collected from the inferior vena cava for detecting blood glucose (BG), serum creatinine (sCr), and blood urea nitrogen (BUN). Both kidneys were quickly removed and rinsed with cold isotonic saline and then weighed. An index of renal hypertrophy was estimated by comparing the wet weight of the left kidney to the body weight. Thereafter, the fresh kidney cortices were dissected. Some of them were placed into liquid nitrogen and stored at −70°C for an assay of tissue oxidative stress parameters, Western blot, and reverse transcription-PCR. The others were fixed in 10% neutralized formalin and embedded in paraffin for morphological and immunohistochemical analysis.
Measurements of Blood Glucose and Renal Function
The levels of sCr and BUN were measured with 7170-A Olympus Biochemistry Test (Olympus Co., Japan). The levels of BG were measured by blood glucose meter OneTouch II (Johnson & Johnson, USA).
Morphological Observation of Renal Pathology
Paraffin-embedded samples were cut at 4μm. Sections were then stained with hematoxylin-eosin (HE). Each HE-stained sample in each group was observed under light microscope. To evaluate glomerular size, digital images of glomeruli areas were obtained from microscopy (magnification, ×400). The glomerular cross-sectional area (AG) was measured in 50 glomerular profiles per rat by using computerized image analysis system (Leica Qwin Standard V2.6, Leica Microsystems, Germany). The results were expressed as mean ± SEM μm2. All measurements were performed blinded.
Assay of Oxidative Stress Parameters
Oxidative stress was evaluated by examining levels of renal malondialdehyde (MDA) and activities of antioxidant enzyme glutathione peroxidase (GSH-Px). MDA and GSH-PX were measured by using commercially available kits according to the manufacturer's protocol (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The data on renal MDA levels and GSH-Px activities were expressed as nmol per milligram protein (nmol/ mg prot), and U per milligram protein (U/mg prot), respectively. The protein content was estimated by the dye binding assay of Bradford assay (Bio-Rad, Hercules, California, USA), with BSA used as a standard.
Western Blot Analysis of LOX-1 Protein Expression in Renal Cortices
In a 1 mL solution containing 10 mmol/L HEPES (PH 7.6), 10 mmol/L KCl, 1.5 mmol/L Mgcl2, 0.5% NP-40, 1 mmol/L DTT, and 0.5 mmol/L PMSF, 50 mg freshly frozen kidney samples were homogenized and lysed for 10 minutes on ice in a 1mL solution containing 10 mmol/L hepes (PH 7.6), 10 mmol/L KCl, 1.5mmol/L Mgcl2, 0.5% NP-40, 1mmol/L DTT, and 0.5mmol/L PMSF. Then, 50 ug of protein were resolved under reducing conditions on 10% separation gels with a 6% stacking SDS- polyacrylamide gel. After transferring onto nitrocellulose membrane, the membranes were hybridized with anti-LOX-1 antibody (Santa Cruz, California, USA; diluted 1:500), subsequently incubated with horseradish peroxidase-conjugated secondary antibody (Zhongshan Biotechnology Co, Ltd., Beijing, China; diluted 1:1000), and developed by chemiluminescence. The membranes were then exposed to x-ray film and subsequently developed immunoreactive bands. Housekeeping protein ß-actin was used as a loading control. Densitometry was performed with gel imaging system (Alphaimager 2200, Pharmacia Biotech Co., USA), and densitometric units were measured as the ratio to ß-actin protein.
Immunohistochemical Analysis of ICAM-1 Protein Expression in Renal Cortices
Goat biotinylation streptavidin-peroxidase complex immunostaining kit (Zhongshan Biotechnology Co, Ltd.) was used, and the procedure was performed according to the manufacture's instructions. Briefly, 4 μm-thick paraffin slices were roasted, deparaffinized and antigen repaired, and then incubated in 0.3%H2O2 to remove endogenous peroxidase activity and 5% BSA in PBS to blocked non-specific staining. Subsequently, the slices were incubated with goat anti-rat ICAM-1/CD54 antibody (R&D Systems, Inc., USA; diluted 1:200) overnight at 4°C and anti-goat biotinylation IgG for 1h at 37°C, and then incubated with peroxidase-conjugated streptavidin for 30 min at 37°C and developed with 3.3-diaminobenzidine to produce a brown color. Sections were then counterstained with hematoxylin and examined under a light microscope.
A computerized image analysis system (Leica Qwin Standard V2.6, Leica Microsystems, Germany) was used to quantify ICAM-1 immunostaining in glomeruli and interstitium. The glomerular ICAM-1 index was calculated using the formula
and fifty glomeruli per animal were evaluated. Immunostaining of ICAM-1 in interstitium was quantified by evaluating the positively stained area of the sections. All measures were under the same light intensity for microscopy.
RT-PCR Assay for LOX-1 and ICAM-1 mRAN Expression in Renal Cortices
Kidney samples were homogenized and total RNA isolated using the Trizol (Shanghai Shengwu Co., Ltd., Shanghai, China). 1 ug of total RNA from each rat sample was reverse-transcribed into cDNA using oligo dT (Shanghai Boya Co., Ltd., Shanghai, China) and MMLV reverse transcriptase (Takara Co., Japan). The cDNA of LOX-1 and ICAM-1 were amplified using TaKaRa PrimeScriptTM one-step RT-PCR kit (Takara Co., Japan). The cycling parameters were at 94°C for 30 seconds, annealing at 61°C for 45 seconds, and extended at 72°C for 30 seconds. The products of PCR-amplified samples were visualized on 1% agarose gels using ethidium bromide. Each specific mRNA band was normalized with a band of relative internal reference ß-actin mRNA. The relative intensity of band of interest was analyzed by gel imaging system (Alphaimager 2200, Pharmacia Biotech Co., USA) and expressed as the ratio to the optical density of ß-actin band. The oligonucleotide primers used are as listed in .
Table 1 Oligonucleotide sequence of primers used for RT-PCR
Statistical Analysis
All data are expressed as mean ± SEM and analyzed using statistical package SPSS 11.0 for Windows. One-way analysis of variance (ANOVA) followed by Dunnett's multiple comparison test was used to determine statistically significant differences among the three groups. A level of p < 0.05 was considered statistically significant.
RESULTS
Clinical and Metabolic Parameters
All STZ-induced diabetic rats showed hyperglycemia, polyuria, and growth retardation. After six weeks of treatment, body weight (BW) of both DM and DM + TAU groups were markedly lower than those of NC group, whereas reduced BW in DM group were unaffected by taurine treatment. BG were considerably elevated to similar levels in DM and DM + TAU groups. There were no significant differences in kidneys weight (KW) among the three groups. But compared with normal control, diabetes was associated with an increase in index of renal hypertrophy (the left KW/ BW), which was markedly reduced by taurine treatment. The levels of BUN and sCr were higher in diabetic rats, whereas taurine treatment significantly reduced BUN and sCr levels compared with the DM group (see ).
Table 2 Clinical and metabolic parameters in the three groups
Parameters of Oxidative Stress
As shows, the renal MDA levels were significantly increased in diabetic rats contrasted with normal control rats (p < 0.01); taurine treatment caused a marked decrease in renal MDA levels compared with the DM group (p < 0.01). The activities of renal GSH-Px in the DM group were significantly lower than those in the NC group (p < 0.01), and taurine treatment restored renal GSH-Px activities compared with the DM group (p < 0.05).
Table 3 Oxidative stress parameters in the three groups of rats
Renal Morphology
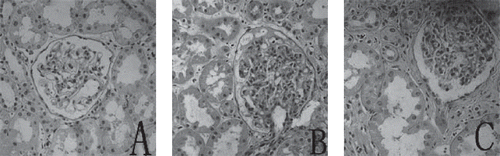
Diabetic rats had an increase in the glomerular surface area (AG) when compared with the values in normal control animals (10698 ± 269 μm2 VS 6636 ± 302 μm2, p < 0.01). Taurine treatment was associated with a reduction of glomerular surface area (7869 ± 387 μm2 vs. 10698 ± 269 μm2, p < 0.01) as compared with the DM group. show representative micrographs of kidney tissue stained with HE from the three groups.
Renal ICAM-1 Protein Expression
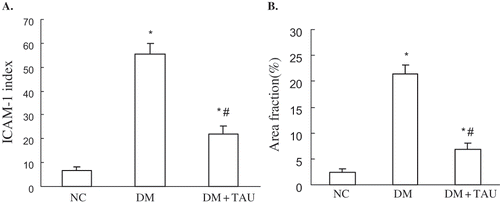
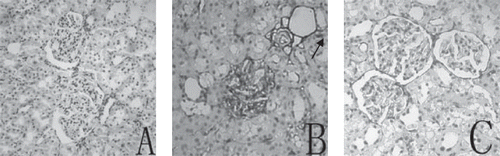
ICAM-1 protein immunostaining was seldom observed in the kidney cortices of NC rats, while significantly higher ICAM-1 immuno reactivities on glomeruli and peritubular capillaries were observed in DM rats, though the administration of taurine in diabetic rats significantly reduced ICAM-1 immuno reactivities (see and ).
Figure 2. Representative micrographs of immunostaining for ICAM-1 in renal tissue from three groups: (A) seldom specific immunoreactivity in the NC group; (B) strong immunoreactivity for ICAM-1 on the glomerulus and peritubular capillaries (arrow) in DM group; (C) significantly reduced immunoreactivity staining of ICAM-1 on the glomerulus and peritubular capillaries (arrow) in DM + TAU group. Original magnification × 200.
Renal LOX-1 Protein Expression
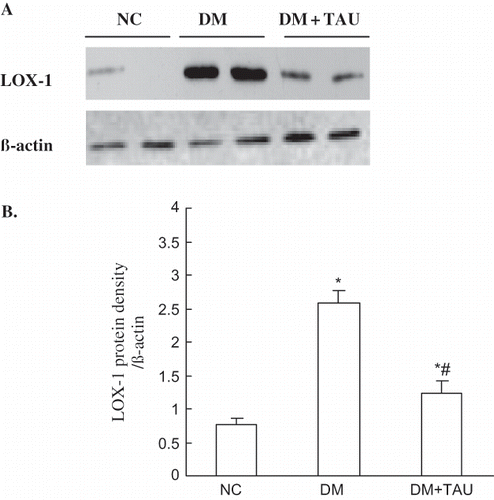
Western blot analysis noted that an increase in the amount of immunoreactive peptide was seen in kidney cortices for diabetic rats compared with that from control animals. Densitometric analysis of the Western blot showed a 3.39-fold increase in the amount of LOX-1 from diabetic rats with contrast to control animals, while taurine treatment reduced this overexpression of LOX-1 protein in diabetic rats by approximately 52% (see ).
Renal ICAM-1 and LOX-1 mRAN Expression
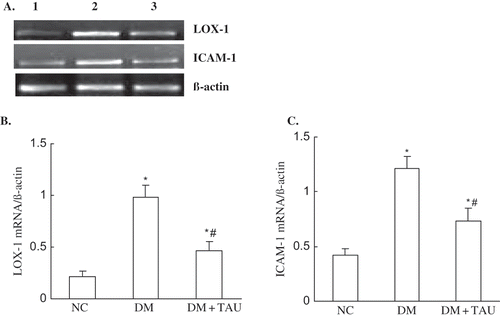
Very low levels could be seen in ICMA-1 mRNA expression and, to a lesser degree, the LOX-1 mRNA expression in kidney cortices tissue of normal control animals, whereas they were increased 4.66-fold and 2.88-fold, respectively, in DM group (p < 0.01, p < 0.01 vs. NC group). Taurine treatment significantly inhibited the upregulated ICAM-1 and LOX-1 mRNA expression in kidney cortices of diabetic rats (p < 0.05, p < 0.01 vs. DM group), as show.
DISCUSSION
At 6 weeks after the injection of STZ, rats had typical characteristics of diabetes mellitus, such as hyperglycemia, polyuria, and emaciate. Also, these diabetic rats exhibited increased levels of BUN and sCr, enhanced index of renal hypertrophy (KW/BW), as well as marked glomeruli hypertrophy in morphology, which all demonstrated the occurrence of early DN in STZ-induced diabetic rats. Taurine treatment significant attenuated these renal pathological changes, despite the absence of glycemia reduction. Simultaneously, the expressions of LOX-1 and ICAM-1 were significantly upregulated in renal cortices of diabetic rats, which were markedly reduced by taurine treatment. We then examined renal oxidative parameters to explain this renoprotective effect and found excessive oxidative stress marked by increased renal MDA levels and decreased GSH-Px activities in diabetic rats, which were also largely ameliorated by taurine treatment. All of these data suggested that taurine showed renoprotective effects in early DN, and that the possible mechanisms were related to a reduction of LOX-1-mediated adhesion molecule ICAM-1 expression in renal cortex.
Endothelial dysfunction induced by oxLDL plays a critical role in the pathogenesis of diabetic vasculopathies.Citation[22] A major risk factor of atherosclerosis, diabetes exerts the atherogenic actions mainly due to the high susceptibility of LDL to oxidative modification in diabetes, and this susceptibility is related to oxidative stress commonly present in diabetes.Citation[23] As a major receptor of oxLDL, endothelial LOX-1 supports the adhesion of monocytes and is upregulated by glycoxidative modifications of lipoproteins in the aortic endothelium of diabetic rats, supporting an important role of LOX-1 in diabetic vasculopathy.Citation[24] LOX-1 induced the activation of the transcription factor NF-kB, which was considered to be one of the major factors involved in ICAM-1 transcription,Citation[6] and such cascade reactions were also present in the renal injury of diabetes.Citation[7] Knockout of ICAM-1 abolished any diabetes-induced increase in glomerular hypertrophy and mesangial matrix expansion, suggesting that ICAM-1 may be a key mediator responsible for renal injury in diabetes.Citation[25] Renal LOX-1 expression was upregulated in renal damages induced by hypertension, ischemia-reperfusion, and hypercholesterolemia, which are all intimately associated with increased oxidative stress. In the present study, we demonstrated the significant upregulation of LOX-1 protein and mRNA in kidney cortices of diabetic rats; moreover, the upregulation of LOX-1 expression was in parallel to the renal expression of ICAM-1, renal dysfunction, and histologic glomerular changes. Simultaneously, the levels of renal MDA were increased and GSH-Px decreased. All of these observations suggested that oxidative stress-induced LOX-1 expression may play an important role in early DN.
Tight glycemia control ameliorates kidney injury in diabetes, but because diabetes-induced oxidative stress is gradually considered to be the main cause of DN by the fact that free radicals, such as lipid peroxides and nitric oxide, are overproduced and antioxidant enzymes such as GSH-Px are decreased in the diabetic kidney.Citation[26] The administration of antioxidants appears to be one of the most reasonable therapeutic approaches. Studies have demonstrated the beneficial effects of antioxidants such as probucol, antiscorbic acid, tocopherol, and melatonin in the complications of diabetes, including DN, via scavenging superoxide, restoring the defective leukocyte–endothelial interaction and enhancing endogenous antioxidant enzyme activities.Citation[15],Citation[27–29] As an endogenous antioxidant, the supplement of taurine has therapeutical effects to diabetes, such as an antihyperglycemic, antihyperlipidemic, and antiinflammatory.Citation[30–32] Furthermore, recent studies have shown the preventive and protective effect of taurine against vascular complications of diabetes.Citation[20],Citation[21] In many studies, taurine also has shown protective effects against renal damages induced by nicotine,Citation[18] tamoxifen,Citation[33] hypertension,Citation[34] and ischemia-reperfusion,Citation[19] all of which were intimately associated with excessive oxidative stress. In the present study, we gave taurine administration to diabetic rats at once after the onset of diabetes, and found that taurine treatment reduced the MDA levels and enhanced GSH-Px activities of renal tissues. Yet it hadn't any effect on blood glucose, which indicated that taurine might play a significant protective effect against early DN mainly via acting as an antioxidant.
A number of studies have showed that LOX-1 expression was upregulated in oxidative stress-induced organ damages, and antioxidant downregulated LOX-1 expression and simultaneously reduced organ injury induced by oxidative stress.Citation[35],Citation[36] In vitro, high glucose-stimulated LOX-1 expression in human aortic endothelial cells was tightly involved in increased oxidative stress, and antioxidants such as vitamin E, vitamin C, or n-acetyl-l-cysteine (NAC) inhibited this high glucose-induced upregulation of LOX-1 expression and the redox-sensitive factor NF-kB.Citation[37] More interestingly, many agents, such as stains, metformin, and pioglitazone, downregulated LOX-1 expression via the reduction of intracellular superoxide radical generation and inhibition of LDL oxidation.Citation[38–40] In the present study, taurine treatment significantly decreased diabetes-induced overexpression of renal LOX-1 protein and gene, simultaneously reduced the renal MDA levels, and restored the renal GSH-Px activities in diabetic rats, suggesting beneficial effects of taurine on early DN may be attributed to the suppression of oxidative stress-induced overexpression of LOX-1 protein and gene in diabetic kidney. The overexpression of ICAM-1, which induces leukocyte infiltration and macrophage recruitment into glomerular, are recognized in DN.Citation[41] LOX-1 plays a critical role on induction of ICAM-1 expression via activating NF-kB, CD40/40L, and PKCε-MAPK signal pathway.Citation[38],Citation[42] In the present study, ICAM-1 expression in the kidney cortices of diabetic rats was largely upregulated, a finding similar to that of previous studies.Citation[8],Citation[9] Taurine treatment reduced ICAM-1 expression on kidney of diabetic rats, accompanied with downregulation of LOX-1 expression, which further suggested that LOX-1 overexpression played an important role in the development of DN and that taurine may downregulate ICAM-1 expression through the inhibition of LOX-1 activation.
In summary, taurine treatment showed protective effects against early renal injury in STZ-induced diabetic rats. Suppression of the role of oxLDL/LOX-1 system and subsequently the LOX-mediated ICAM-1 expression by its antioxidative property may be one possible mechanism for the renoprotective effects of taurine. Taurine administration might be a good option for the therapy of diabetic patients to reduce oxidative stress and associated renal injury. Continued investigations should be encouraged regarding its effects on renal protection when used at different doses and time points, by different administration routes, and in different age stages.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
ACKNOWLEDGMENT
The present study was supported by National Dr Site Foundation of Chinese Education Ministry (No. 200504 22050) and Excellent Adult and Young Scientist Science Foundation of Shandong Province (No. 2006B5003065).
REFERENCES
- Bryla J, Kiersztan A, Jagielski AK. Promising novel approaches to diabetes mellitus therapy: Pharmacological, molecular and cellular insights. Eur Citiz Qual Life. 2003; 1: 137–161
- Ha H, Kim KH. Pathogenesis of diabetic nephropathy: The role of oxidative stress and protein kinase C. Diabetes Res Clin Prac. 1999; 45: 147–151
- Ujihara N, Sakka Y, Takeda M, Hirayama M, Ishii A, Tomonaga O, Babazono T, et al. Association between plasma oxidized low-density lipoprotein and diabetic nephropathy. Diabetes Res Clin Pract. 2002; 58: 109–114
- Chen M, Masaki T, Sawamura T. LOX-1, the receptor for oxidized low-density lipoprotein identified from endothelial cells: Implications in endothelial dysfunction and atherosclerosis. Pharmacol Ther. 2002; 95: 89–100
- Cominacini L, Pasini AF, Garbin U, Davoli A, Tosetti ML, Campagnola M, et al. Oxidized low density lipoprotein (ox-LDL) binding to ox-LDL receptor-1 in endothelial cells induces the activation of NF-kappaB through an increased production of intracellular reactive oxygen species. J Biol Chem. 2000; 275: 12633–12638
- Guijarro C, Egido J. Transcription factor-kappa B (NF-kappaB) and renal disease. Kidney Int. 2001; 59: 415–424
- Usui H, Shikata K, Matsuda M, Okada S, Ogawa D, Yamashita T, et al. HMG-CoA reductase inhibitor ameliorates diabetic nephropathy by its pleiotropic effects in rats. Nephrol Dial Transplant. 2003; 18: 265–272
- Wu Y, Wu G, Qi X, Lin H, Qian H, Shen J, et al. Protein kinase C β inhibitor LY333531 attenuates intercellular adhesion molecule-1 and monocyte chemotactic protein-1 expression in the kidney in diabetic rats. J Pharmacol Sci. 2006; 101: 335–343
- Nagase M, Kaname S, Nagase T, Wang G, Ando K, Sawamura T, et al. Expression of LOX-1, an oxidized low-density lipoprotein receptor, in experimental hypertensive glomerulosclerosis. J Am Soc Nephrol. 2000; 11: 1826–1836
- Kosaka H, Yoneyama H, Zhang L, Fujii S, Yamamoto A, Igarashi J. Induction of LOX-1 and iNOS expressions by ischemia-reperfusion of rat kidney and the opposing effect of L-arginine. FASEB J. 2003; 17: 636–643
- Wilson SH, Chade AR, Feldstein A, Sawamura T, Napoli C, Lerman A, et al. Lipid-lowering-independent effects of simvastatin on the kidney in experimental hypercholesterolaemia. Nephrol Dial Transplant. 2003; 18: 703–709
- Ueno T, Kaname S, Takaichi K, Nagase M, Tojo A, Onozato ML, et al. LOX-1, an oxidized low-density lipoprotein receptor, was upregulated in the kidneys of chronic renal failure rats. Hypertens Res. 2003; 26: 117–122
- Reichard P, Neilsson BY, Roseuqvist U. The effect of intensive treatment of diabetes on the development and progression of long-term complication in insulin-dependent diabetes mellitus. N Engl J Med. 1993; 329: 977–986
- Stackhous S, Miller PI, Park SK, Mever TWL. Reversal of glomerular hyperfiltration and renal hypertrophy by blood glucose normalization in diabetic rats. Diabetes. 1990; 39: 985–995
- Winiarska K, Fraczyk T, Malinska D, Drozak J, Bryla J. Melatonin attenuates diabetes-induced oxidative stress in rabbits. J Pineal Res. 2006; 40: 168–176
- Chang JW, Lee EK, Kim TH, Min WK, Chun S, Lee KU, et al. Effects of alpha-lipoic acid on the plasma levels of asymmetric dimethylarginine in diabetic end-stage renal disease patients on hemodialysis: A pilot study. Am J Nephrol. 2007; 27: 70–74
- Egan BM, Abdih H, Kelly CJ, Condron C, Bouchier-Hayes DJ. Effect of intravenous taurine on endotoxin-induced acute lung injury in sheep. Eur J Surg. 2001; 167: 575–580
- Sener G, Sehirli O, Ipci Y, Cetine S, Cikler E, Gedik N, et al. Protective effects of taurine against nicotine- induced oxidatvie damage of rat urinary bladder and kidney. Pharmacology. 2005; 74: 37–44
- Guz G, Oz E, Lortlar N, Ulusu NN, Nurlu N, Demirogullari B, et al. The effect of taurine on renal ischemia-reperfusion injury. Amino Acids. 2007; 32: 405–411
- Li C, Cao L, Zeng Q, Liu X, Zhang Y, Dai T, et al. Taurine may prevent diabetic rats from developing cardiomyopathy also by downregulating angiotensin II type2 receptor expression. Cardiovascular Drugs and Therapy. 2005; 18: 105–112
- Li F, Abatan OI, Kim H, Burnett D, Larkin D, Obrosova IG, et al. Taurine reverses neurological and neurovascular deficits in Zucker diabetic fatty rats. Neurobiology of Disease. 2006; 22: 669–676
- Tan KC, Ai VH, Chow WS, Chau MT, Leong L, Lam KS. Influence of low density lipoprotein (LDL) subfraction profile and LDL oxidation on endothelium-dependent and independent vasodilation in patients with type 2 diabetes. J Clin Endocrinol Metab. 1999; 84: 3212–3216
- de Castro SH, Castro-Faria-Neto HC, Gomes MB. Association of postprandial hyperglycemia with vitro LDL oxidative in no-smoking patients with Type 1 diabetes—a cross-sectional study. Rev Diabet Stud. 2005; 2: 157–164
- Chen M, Nagase M, Fujita T, Narumiya S, Masaki T, Sawamura T, et al. Diabetes enhances lectin-like oxidized LDL receptor-1 (LOX-1) expression in the vascular endothelium: Possible role of LOX-1 ligand and AGE. Biochem Biophys Res Commun. 2001; 287: 962–968
- Okada S, Shikata K, Matsuda M, Ogawa D, Usui H, Kido Y, Nagase R, et al. Intercellular adhesion molecule-1-deficient mice are resistant against renal injury after induction of diabetes. Diabetes. 2003; 52: 2586–2593
- Anwar MM, Meki AR. Oxidative stress in streptozotocin-induced diabetic rats: Effects of garlic oil and melatonin. Comparative Biochemistry and Physiology, Part A. 2003; 135: 539–547
- Zanardo RC, Costa Cruz JW, Oliveira MA, Fortes ZB. Probucol restores the defective leukocyte-endothelial interaction in experimental diabetes. Eur J Pharmacol. 2003; 478: 211–219
- Zanardo RC, Costa Cruz JW, Oliveira MA, Fortes ZB. Ascorbic acid supplementation restores defective leukocyte-endothelial interaction in alloxan-diabetic rats. Diabetes Metab Res Rev. 2003; 19: 60–68
- Dhein S, Kabat A, Olbrich A, Rosen P, Schroder H, Mohr FW. Effect of chronic treatment with vitamin E on endothelial dysfunction in a type I in vivo diabetes mellitus model and in vitro. J Pharmacol Exp Ther. 2003; 305: 114–122
- Tenner TE, Jr, Zhang XJ, Lombardini JB. Hypoglycemic effects of taurine in the alloxan- treated rabbit: A model for type 1 diabetes. Adv Exp Med Biol. 2003; 526: 97–104
- Casey RG, Gang C, Joyce M, Bouchier-Hayes DJ. Taurine attenuates acute hyperglycaemia-induced endothelial cell apoptosis, leucocyte-endothelial cell interactions and cardiac dysfunction. J Vasc Res. 2007; 443: 1–9
- Murakami S, Kondo Y, Sakurai T, Kitajima H, Nagate T. Taurine suppresses development of atherosclerosis in Watanabe heritable hyperlipidemic (WHHL) rabbits. Atherosclerosis. 2002; 163: 79–87
- Tabassum H, Parvez S, Rehman H, Dev Banerjee B, Siemen D, Raisuddin S. Nephrotoxicity and its prevention by taurine in tamoxifen induced oxidative stress in mice. Human & Experimental Toxicology. 2007; 26: 509–518
- Chiba Y, Ando K, Fujita T. The protective effects of taurine against renal damage by salt loading in Dahl salt-sensitive rats. J Hypertens. 2002; 20: 2269–2274
- Li D, Williams V, Liu L, Chen H, Sawamura T, Antakli T, et al. LOX-1 inhibition in myocardial ischemia-reperfusion injury: Modulation of MMP-1 and inflammation. Am J Physiol Heart Circ Physiol. 2002; 283: H1975–H1801
- Muscoli C, Sacco I, Alecce W, Palma E, Nistico R, Costa N, et al. The protective effect of superoxide dismutase mimetic M40401 on balloon injury-related neointima formation: Role of the lectin-like oxidized low-density lipoprotein receptor-1. J Pharmacol Exp Ther. 2004; 311: 44–50
- Li L, Sawamura T, Renier G. Glucose enhances endothelial LOX-1 expression role for LOX-1 in glucose-induced human monocyte adhesion to endothelium. Diabetes. 2003; 52: 1843–1850
- Li D, Chen H, Romeo F, Sawamura T, Saldeen T, Mehta JL. Statins modulate oxidized low-density lipoprotein-mediated adhesion molecule expression in human coronary artery endothelial cells: Role of LOX-1. J Pharmacol Exp Ther. 2002; 302: 601–605
- Mehta JL, Hu B, Chen J, Li D. Pioglitazone inhibits LOX-1 expression in human coronary artery endothelial cells by reducing intracellular superoxide radical generation. Arterioscler Thromb Vasc Biol. 2003; 23: 2203–2208
- Ouslimani N, Mahrouf M, Peynet J, Bonnefont-Rousselot D, Cosson C, et al. Metformin reduces endothelial cell expression of both the receptor for advanced glycation end products and lectin-like oxidized receptor 1. Metabolism Clinical and Experimental. 2007; 56: 308–313
- Sugimoto H, Shikata K, Hirata K, Akiyama K, Matsuda M, Kushiro M, et al. Increased expression of intercellular adhesion molecule-1 (ICAM-1) in diabetic rat glomeruli: Glomerular hyperfiltration is a potential mechanism of ICAM-1 upregulation. Diabetes. 1997; 46: 2075–2081
- Kobayashi N, Hara K, Tojo A, Onozato ML, Honda T, Yoshida K, et al. Eplerenone shows renoprotective effect by reducing LOX-1-mediated adhesion molecule, PKCε-MAPK-p90RSK, and rho-kinase pathway. Hypertension. 2005; 45: 538–544