Abstract
Objective. Hemodialysis is a common therapeutic strategy for patients with end stage renal failure. During the hemodialytic process, the neutrophils are activated (neutrophil burst) due to the hemoincompatibility induced by hemodialysis. As a result, the activated neutrophils release reactive oxygen species (ROS), such as hydrogen peroxide, singlet oxygen, and hypochlorite, into the bloodstream and cause oxidative damage. Methods. This study investigated the antioxidant alteration of plasma in uremic patients undergoing hemodialysis by chemiluminescent analysis. The antioxidant capacities of plasma in scavenging hydrogen peroxide, singlet oxygen, and hypochlorite were investigated in this experiment. In addition, investigation of the ferric-reducing ability of plasma (FRAP) would be covered in this study as well. Results. This study found that after hemodialysis, the antioxidant capacities of plasma in scavenging hydrogen peroxide, singlet oxygen, and hypochlorite decreases 7.9%, 18.8%, and 18.9%, respectively. Moreover, the FRAP is reduced by 56%. We speculate that the loss of dialyzable solutes (such as uremic solutes and antioxidants with small molecular weight) in plasma resulted in its decrease in antioxidant capacity. Conclusion. We therefore suggest that the supplement of antioxidants with small molecular weight is capable of regaining antioxidant defense in plasma and preventing oxidative damage induced by hemodialysis.
INTRODUCTION
Hemodialysis is a common therapeutic strategy for patients at the end stage of renal failure. Although hemodialysis is capable of removing metabolic wastes (uremic toxins) through diffusion, it causes the imbalance of homeostasis due to the hemoincompatibility induced by the contact between blood and artificial hemodialyzer. The production of oxidative stress is one of the results induced by hemoincompatibility.Citation[1],Citation[2] When plasma oxidative stress is elevated, the risk of complications associated with oxidative stress, such as atherosclerosis, inflammation, immunodysfunction, and cancer, is also increased.Citation[3],Citation[4]
The most acceptable mechanism for the production of oxidative stress induced by hemoincompatibility is “neutrophil burst.”Citation[5],Citation[6] The hemoincompatibility induced by hemodialysis activates the neutrophils, which then release reactive oxygen species (ROS) such as hypochlorite, hydrogen peroxide, and singlet oxygen into the bloodstream. These ROS can react with plasma biomolecules (such as proteins, lipids, and cell membrane materials) and cause oxidative damage on these biomolecules. As such, the monitor of alteration of oxidative stress in uremic patients becomes an important issue during hemodialysis.
The determination of oxidative metabolites in plasma (such as malondialdehyde, protein carbonyls, and oxidized LDL) is the most common method in monitoring the alteration of oxidative stress in uremic patients undergoing hemodialysis.Citation[7],Citation[8] This is primarily because the high concentration of oxidative metabolites in plasma indicates a high risk of oxidative damage.
Another method in determining oxidative stress in uremic patients is to investigate the alteration of plasma antioxidant capacity, including the ferric-reducing ability of plasma (FRAP) or its oxygen radical absorbance capacity (ORAC).Citation[9],Citation[10] The decrease of plasma antioxidant capacity leads to a high risk in ROS attack. Moreover, chemiluminescent analysis is also used in determining plasma oxidative stress.Citation[11] The oxidants existing in plasma react with light-emitting materials (luminol or lucigenin), which then release ultra-weak luminescence that can be detected by chemiluminescent analyzer. The intensity of chemiluminescence is proportional to the concentration of plasma oxidants.
This study aims to investigate the alteration of plasma antioxidant capacities in scavenging singlet oxygen, hypochlorite, and hydrogen peroxide (the three ROS mainly released from neutrophil burst) in uremic patients undergoing hemodialysis by chemiluminescent analysis. This study also covers the antioxidant capacities of some food extracts in scavenging the above-mentioned ROS by chemiluminescent analysis.
METHODS
Chemicals and Reagents
In this study, acetonitrile and ethanol were purchased from Mallinckrodt Baker, Inc. (Phillipsburg, New Jersey, USA), while phosphate buffered saline (PBS) was purchased from Invitrogen Corporation (Grand Island, New York, USA). Other chemicals and reagents used in this study were from Sigma-Aldrich Inc. (St. Louis, Missouri, USA).
Experimental Subjects
Sixteen healthy volunteers and twenty uremic patients were involved in this experiment. The uremic patients contained both genders (11 males and 9 females) with averaged hemodialytic duration for 89 ± 24 months. The hemodialytic processes for the uremic patients followed the standard operation procedures (SOP) in Formosan Blood Purification Foundation (Taipei, Taiwan). Healthy volunteers (nine males and seven females) were selected according their laboratory blood tests. The plasma samples collected from uremic and healthy volunteers were stored at −80°C before use.
Singlet Oxygen-Scavenging Potential
The first step of this experiment was to produce singlet oxygen in chemiluminescent analyzer by mixing Na2CO3 buffer, H2O2 and acetonitrile in basic condition.Citation[12] In the experiment, 0.5 mL of Na2CO3 buffer (100 mM), 0.05 mL of H2O2 (1.7%), and 0.05 mL of diluted plasma or analytes were added into the chemiluminescent analyzer (CLA-FS1, Tohoku Electronic Industrial Co. Ltd., Rifu Town, Miyagi, Japan). After mixing for 20 seconds, 0.1 mL of luminol solution (20 mg of luminol in 20 mL of 30% acetonitrile) was added by injection. Intensive chemiluminescence was detected in this step due to singlet oxygen production. The chemiluminescent measurement was terminated after 60 seconds. During the experiment, the distilled water acted as blank and trolox (6–Hydroxy–2,5,7, 8–tetramethylchroman–2–carboxylic acid, a water-soluble vitamin E-like antioxidant) as standard. The scavenging potentials of singlet oxygen for the analytes were expressed as trolox equivalents (TE).
Hypochlorite-Scavenging Potential
The experimental protocol was modified from Li et al.Citation[13] In this experiment, 1 mL of distilled water, 0.02 mL of NaOCl (0.037%), and 0.1 mL of diluted plasma or analytes were added into the chemiluminescent analyzer. After mixing for 20 seconds, 0.1 mL of luminol solution (44 mg of luminol in 10 mL of 0.01 N sodium hydroxide) was added by injection. Intensive chemiluminescence was observed in this step. The chemiluminescent measurement was terminated after 90 seconds. During the experiment, the distilled water acted as blank and trolox as standard. The scavenging potentials of hypochlorite for the analytes were expressed as trolox equivalents.
Hydrogen Peroxide-Scavenging Potential
The chemiluminescence induced by H2O2 can be measured by mixing H2O2 and lucigenin in tris buffer.Citation[14] In this study, 0.1 mL of H2O2 (1.7%), 1 mL of tris buffer (50 mM, pH = 7.2), and 0.1 mL of diluted plasma or analytes were added into the chemiluminescent analyzer. After mixing for 20 seconds, 0.1 mL of lucigenin solution (1.96 mM) was added by injection. Intensive chemiluminescence was measured in this step. The chemiluminescent measurement was terminated after 90 seconds. During the experiment, the distilled water acted as blank and trolox as standard. The scavenging potentials of hydrogen peroxide for the analytes were expressed as trolox equivalents.
Ferric-Reducing/Antioxidant Power (FRAP) Assay
The FRAP assay for this experiment was modified from Othman et al.Citation[15] The FRAP reagent was prepared by mixing acetate buffer (246.1 mg of sodium acetate in 10 mL of 10% acetic acid), TPTZ solution (31.23 mg of TPTZ and 0.044 ml of 37% HCl in 10 mL of distilled water), and FeCl3 solution (54.06 mg of FeCl3·6H2O in 10 mL of distilled water) in a ratio of 10:1:1. The FRAP reagent (0.5 mL), distilled water (0.05 mL), and 0.016 mL of analytes were transferred into vials and then incubated at room temperature. After incubation for 4 minutes, the absorbance (λ = 593 nm) was measured for all vials. The distilled water acted as blank, and the FRAP data for the analytes were expressed as FeSO4 equivalents.
Blood Test for Uremic Patients
The quantification of plasma uremic markers (including blood urea nitrogen, creatinine, uric acid, and β2-MG) was determined by IMMULITE instrument (DPC Cirrus Inc., Los Angeles, California, USA).
Statistical Analysis
All experimental data were expressed as mean ± standard deviation (SD). Data analysis was carried out by using paired t-test. Statistical difference was to be considered at the level of p < 0.05.
RESULTS
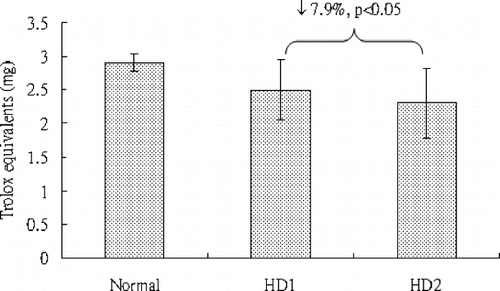
Hydrogen peroxide oxidizes lucigenin, which releases ultra-weak luminescence that is detected by the chemiluminescent analyzer. As such, the intensity of chemiluminescence is proportional to the concentration of hydrogen peroxide. Moreover, the addition of plasma in hydrogen peroxide-lucigenin reaction leads to a decrease in chemiluminescent intensity because the antioxidants existing in plasma scavenge hydrogen peroxide. Therefore, the reduction of chemiluminescence positively correlates with plasma antioxidant capacity in scavenging hydrogen peroxide. shows the antioxidant capacity of plasma in scavenging chemiluminescence induced by hydrogen peroxide-lucigenin reaction. The antioxidant capacities of pre-hemodialytic (HD1) and post-hemodialytic (HD2) plasma samples are 2.495 ± 0.452 TE and 2.298 ± 0.524 TE, respectively. By comparison of HD1 and HD2, the antioxidant capacity of plasma in scavenging hydrogen peroxide is reduced by 7.9% (p < 0.05) in the uremic patients after hemodialysis.
Similar results can be observed in and . and show the antioxidant capacities of plasma in scavenging chemiluminescence induced by hypochlorite and singlet oxygen, respectively. In , the antioxidant capacities for HD1 and HD2 are 0.062 ± 0.009 TE and 0.051 ± 0.011 TE. In , the antioxidant capacities for HD1 and HD2 are 0.682 ± 0.071 TE and 0.554 ± 0.089 TE. The antioxidant capacities of plasma in scavenging hypochlorite and single oxygen are respectively reduced 18.9% (p < 0.05) and 18.8% (p < 0.05) in uremic patients after hemodialysis.
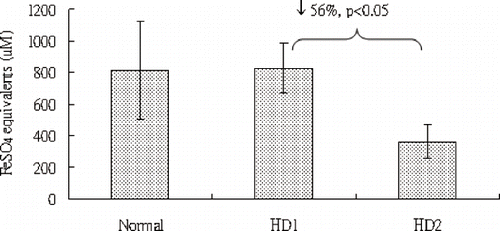
In addition to chemiluminescent analysis, we also investigated the FRAP in uremic patients with hemodialysis. The FRAP assay evaluates the electron-donating ability of plasma. Antioxidants are known for easily donating electrons to terminate chain reactions induced by free radicals. This is one reason why antioxidants are capable of scavenging free radicals. Therefore, the FRAP value is proportional to the antioxidant capacity of plasma. In , the FRAP values for HD1 and HD2 are 827 ± 161 and 363 ± 109, respectively. This means that the plasma FRAP (antioxidant capacity) has decreased 56% (p < 0.05) in uremic patients after hemodialysis.
shows the results of blood test for uremic patients undergoing hemodialysis. We can observe the clearance percentage of uremic solutes including blood urea nitrogen (73.93%), creatinine (69.67%), uric acid (79.63%), and β2-MG (55.44%).
Table 1 Blood test for uremic patients undergoing hemodialysis
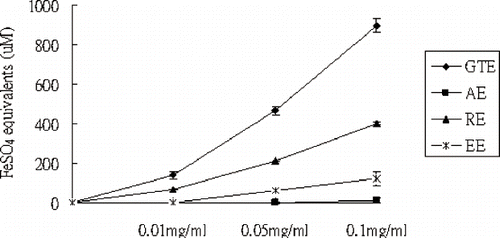
shows the chemiluminescent analysis of some food extracts in scavenging hydrogen peroxide, hypochlorite, and singlet oxygen. It indicates that the green tea extract (GTE) exhibits the most excellent capacities in scavenging above three ROS compared to the apple extract (AE), Rhodiola rosea extract (RE) and Eleutherococcus senticosis extract (EE). In addition, shows the FRAP assay for the four extracts. In high concentration (0.1 mg/mL), the FRAP of the four extracts follow the order of GTE (897 ± 37) > RE (402 ± 16) > EE (121 ± 11) > AE (13 ± 1). In low concentration (0.01 mg/mL), the order of the FRAP for the four extracts is GTE (138 ± 20) > RE (66 ± 3) > EE (undetectable) ≅ AE (undetectable).
Table 2 ROS scavenging capacities of some food extracts
DISCUSSION
Hydrogen peroxide, hypochlorite, and singlet oxygen are the main ROS released from neutrophil burst induced by hemodialysis. Based on our experimental results, the antioxidant capacities of plasma in scavenging the above three ROS were decreasing in uremic patients after hemodialysis. We speculate that some antioxidant dialyzable solutes were removed during hemodialysis. For instance, uric acid is studied to exhibit antioxidant property in plasma. In , we can find that the plasma level of uric acid depletes by 79.63% after patients with hemodialysis. The loss of antioxidant dialyzable solutes resulted in the defect of plasma antioxidant defense.Citation[16–18] As such, this may be the reason why hemodialysis leads to a decrease of plasma antioxidant capacity in uremic patients. The alteration of plasma FRAP in also supports this explanation.
Results of the experiment point out that the reduction of plasma antioxidant capacities in scavenging the above three ROS is in the order of hydrogen peroxide (7.9%) > singlet oxygen (18.8%) ≅ hypochlorite (18.9%). The reduction of plasma antioxidant capacity in scavenging hydrogen peroxide is less than the other two ROS. This may be associated with the catalase level in plasma. The catalase, an enzyme that converts hydrogen peroxide into water and oxygen in plasma, has a molecular weight of about 230 kDa. The hemodialytic process is not able to remove the solute with such a large molecular weight (averaged pore size of hemodialyzer is less than 66 kDa). Therefore, the catalase level in plasma does not alter dramatically in uremic patients after hemodialysis. On the other hand, there is no specific plasma enzyme in scavenging hypochlorite and singlet oxygen. Instead, the removal of the two ROS depends on small antioxidants existing in plasma. This may be a reason why the alteration of antioxidant capacity in scavenging hydrogen peroxide is less than the other two ROS.
In the experiment, the chemiluminescent analysis of the antioxidant capacities in scavenging the above three ROS as well as the FRAP assay for some food extracts were also investigated. Results show that GTE exhibits the most excellent capacities in scavenging the above three ROS and the best FRAP compared to RE, AE, and EE. This may be because GTE contains a high level of polyphenol ingredients (the order of polyphenol ingredients: GTE > RE > AE > EE, data not shown). Further, Hsu et al. Citation[19] pointed out that supplement of GTE reduced hemodialysis-enhanced production of ROS. These data support the idea that the supplement of GTE may assist uremic patients in avoiding oxidative stress induced by hemodialysis.
In summary, the supplement of antioxidants with small molecular weight (such as GTE in this article) is capable of replenishing the lost dialyzable solutes in uremic patients during hemodialysis. This can reduce oxidative damage induced by the neutrophil burst and eventually improve the quality of life of uremic patients undergoing hemodialysis.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Horl WH. Hemodialysis membranes: Interleukins, biocompatibility and middle molecules. J Am Soc Nephrol. 2002; 13: S62–S71
- Moreun M, Cristol JP, Canaud B. Why hemodialysis patients are in a prooxidant state: What could be done to correct the pro/antioxidant imbalance. Blood Purif. 2000; 18: 191–199
- Jofre R, Rodriguez-Benitez P, Lopez-Gomez JM, Perez Garcia R. Inflammatory syndrome in patients on hemodialysis. J Am Soc Nephrol. 2006; 17: S274–S280
- Himmelfarb J, Hakim RM. Oxidative stress in uremia. Curr Opin Nephrol Hypertens. 2003; 12: 593–598
- Hodkova M, Dusilova-Sulkova S, Skalicka A, Kalousova M, Zima T, Bartunkova J. Influence of parenteral iron therapy and oral vitamin E supplementation on neutrophil respiratory burst in chronic hemodialysis patients. Ren Fail. 2005; 27: 135–141
- Hernandez MR, Galan AM, Cases A, Lopez-Pedret J, Pereira A, Tonda R, Bozzo J, Escolar G, Ordinas A. Biocompatibility of cellulosic and synthetic membranes assessed by leukocyte activation. Am J Nephrol. 2004; 24: 235–241
- Mimic-Oka J, Savic Radojeric A, Pljesa Eriegovac M, Opacic M, Simic T, Dimkovic N, Simic DV. Evaluation of oxidative stress after repeated intravenous iron supplementation. Ren Fail. 2005; 27: 345–151
- Erdogan A, Unluceric Y, Turkmen A, Kurn A, Cetin O, Bekpinar S. The evaluation of oxidative stress in patients with chronic renal failure. Clin Chin Acta. 2002; 322: 157–161
- Gerardi G, Usberti M, Martini G, Albertini A, Sugherini L, Dompella A, Di LD. Plasma total antioxidant capacity in hemodialyzed patients and its relationships to other biomarkers of oxidative stress and lipid peroxidation. Clin Chem Lab Med. 2002; 40: 104–110
- Balakrishnan VS, Blumberg J, Pereira BJ, Jaber BL. Antioxidant and oxidative stress induces in dialysis-dependent acute renal failure. Bloob Purif. 2003; 21: 213–219
- Aqatsuma S, Negoshi T, Kobayashi M, Usa M, Watanabe H, Sekino H, Inaba H. Hydroxyl radical-induced characteristic chemiluminescent spectra from plasma of hemodialysis patients. Clin Chem. 1992; 38: 48–55
- Lu J, Lau C, Lee MK, Kai M. Simple and convenient chemiluminescence method for the determination of melatonin. Anal Chim Acta. 2002; 455: 193–198
- Li J, Dasgupta PK. Chemiluminescence detection with a liquid core waveguide determination of ammonium with electrogenerated hypochlorite based on luminol-hypochlorite reaction. Anal Chim Acta. 1999; 398: 33–39
- Yeung SY, Lau WH, Huang CS, Lin CP, Chan CP, Chang MC, Jeng JH. Scavenging property of three cresol isomers against H2O2, hypochlorite, superoxide and hydroxyl radicals. Food Chem Toxicol. 2002; 40: 103–113
- Othman A, Ismail A, Ghani NA, Adenan I. Antioxidant capacity and phenolic content of cocoa beans. Food Chem. 2007; 100: 1523–1530
- Dursun D, Ozben T, Suleymanlar G, Dursun B, Yakupoglu G. Effect of hemodialysis on the oxidative stress and antioxidants. Clin Chem Lab Med. 2002; 40: 1009–1013
- Nakayama K, Terawaki H, Nakayama M, Iwabuchi M, Sato T, Ito S. Reduction of serum antioxitive capacity during hemodialysis. Clin Exp Nephrol. 2007; 11: 218–224
- Soska V, Ciz M, Kubala L, Sobotova D, Lojek A. Phagocyte-derived oxidants and plasma antioxidants in hemodialysis patients. Scand J Clin Lab Invest. 2007; 67: 343–351
- Hsu SP, Wu MS, Yang CC, Huang KC, Liou SY, Hsu SM, Chien CT. Chronic green tea supplementation reduces hemodialysis-enhanced production of hydrogen peroxide and hypochlorous acid, atherosclerotic factors, and inflammatory cytokines. Am J Clin Nutr. 2007; 86: 1539–1547