Abstract
Ischemia/reperfusion injury, which is commonly seen in the field of renal surgery or transplantation, is a major cause of acute renal failure. The objective of the present study was to examine the role of nebivolol in modulating peroxynitrite species-induced inflammation and apoptosis after renal warm ischemia/reperfusion injury in rats. The present study was designed to investigate the effects of nebivolol on the renal warm ischemia/reperfusion injury in rats treated with the nitric oxide synthase inhibitor, Nω-nitro-L-arginine methyl ester. After right nephrectomy, nebivolol was administered for 15 days. On the 16th day, ischemia was induced in contra lateral kidney for 45 min, followed by reperfusion for 24 hr. Renal function, inflammation, and apoptosis were estimated at the end of 24 hr reperfusion. Nebivolol improved the renal dysfunction and reduced inflammation and apoptosis after renal ischemia/reperfusion injury. In conclusion, nebivolol shows potent anti-apoptotic and anti-inflammatory properties due to its NO-releasing property. These findings may have major implications in the treatment of human ischemic acute renal failure.
INTRODUCTION
Ischemia/reperfusion (I/R) is an important cause of organ dysfunction, often causing high mortality.Citation[1] Ischemic cell injury in the kidney occurs during cardiovascular surgery, renal transplantation, as well as the early allograft rejection subsequent to renal transplantation.Citation[2] Recent studies clearly indicate that nitric oxide is fundamentally involved in the regulation of renal hemodynamics and homeostasis.Citation[3] Moreover, it has been shown that reactive oxygen species (ROS) increase in the areas of ischemia and reperfusion.Citation[4] ROS avidly react with nitric oxide (NO) and produce highly reactive nitrogen species (peroxynitrite), which can lead to functional NO deficiency. Peroxynitrite is an important agent that causes lipid peroxidation of vascular membranes.Citation[5],Citation[6] Studies proved that functional deficiency of NO leads to increased renal sympathetic nerve activity, and this sympathetic activation increases β receptor activities in the body.Citation[7] In addition, studies have shown that the β adrenoceptor present in kidney were exclusively of the β 1 type.Citation[8]
Nebivolol 1-(6-fluorochroman-2-yl)-2-[[2-(6-fluorochroman-2-yl)-2-hydroxy-ethyl] amino] ethanol is a third-generation β-blocker having highly selective β1 adrenergic receptor blockade.Citation[9] It is reported to possess antihypertensive, anti-oxidant activity, and also reduces renal fibrosis and prevents endothelial dysfunction.Citation[10],Citation[11]
So far, no report has proved that treatment with nebivolol could improve the survival rate after renal warm I/R injury. The present study examined whether treatment with nebivolol improve the survival rate in a renal warm I/R injury using a rat model, as renal I/R injury is due to the over-activation of sympathetic β1 receptor activity that ultimately leads to a functional deficiency of NO.
MATERIALS AND METHODS
Sprague-Dawley rats of either sex with body weight between 230 and 260 gms were housed in an air-conditioned room with 12-h light and dark cycles, with free access to food and water ad libitum during the experiments. Experimental protocols were approved by the institutional animal ethics committee. All of the experiments were conducted as per norms of CPCSEA.
Grouping of Animals
The rats were divided into five groups each consisting of six animals:
Group 1. Animals served as sham-operated (underwent all surgical procedures without ischemia reperfusion);
Group 2. After right nephrectomy on day 1, vehicle (0.5 % sodium CMC) was administered for 15 days; on day 16, ischemia was produced in the left kidney for 45 min, followed by reperfusion of 24 hr (I/R control);
Group 3. After right nephrectomy on day 1, L-NAME (10 mg/kg/day, i.p.)Citation[12] was administered for 15 days; on day 16, ischemia was produced in the left kidney for 45 min, followed by reperfusion of 24 hr (I/R+L-NAME);
Group 4. After right nephrectomy on day 1, L-NAME (10 mg/kg/day, i.p.) and nebivolol (2 mg/kg/day, p.o.)Citation[13] were administered for 15 days; on day 16, ischemia was produced in the left kidney for 45 min, followed by reperfusion of 24 hr (I/R+L-NAME+Neb); and
Group 5. After right nephrectomy on day 1, L-NAME (10 mg/kg/day, i.p.) and atenolol (20 mg/kg/day, p.o.)Citation[14] were administered for 15 days. On day 16, ischemia was produced in the left kidney for 45 min, followed by reperfusion of 24 hr (I/R+L-NAME + Atn).
Surgical Procedure
Right nephrectomy was performed through a right flank incision (2 cm) under general anesthesia, ketamine (100 mg/kg, i.p.). After right nephrectomy, several treatments were given as mentioned previously for 15 days. On day 16, ischemia was produced in the left kidney by performing a left flank incision and dissecting the left renal pedicle to expose the renal vessels. Nontraumatic vascular clamps were used to stop blood flow (in artery and vein) for 45 min. Reperfusion was established by removing the clamp after 45 min ischemia. The abdominal wall (muscular layer and skin) was closed with 4.0 mononylon suture. At the end of reperfusion period (after 24 hr), blood samples were collected and used for the estimation of renal function (BUN and creatinine) and TNF-α (marker of inflammation). The abdomen was opened, and the kidneys were harvested for the estimation of MPO activity (marker of inflammation), biomarkers of oxidative stress, nitrite level, and histopathology and cell necrosis.
Measurement of Systolic Blood Pressure
During treatment schedule, systolic blood pressure (SBP) was measured from tail vein by tail cuff instrument (LE 5002, Storage Pressure Meter, Latica) on day 1 (on the day of unilateral nephrectomy), 7, 15 (before ischemia), and 17 (after 24 hr of reperfusion).
Serum Estimation for Renal Function (BUN and Creatinine) and TNF-α (Marker of Inflammation)
Renal Function
Serum samples were assayed for blood urea nitrogen (Jaffe`s method) and serum creatinine (DAM method) by using standard diagnostic kits (Span Diagnostics, Gujarat, India).
TNF-α Quantification by ELISA
Levels of TNF-α in serum were determined using an enzyme-linked immunosorbent assay (ELISA) (Endogen, mouse TNF-α kit, Pierce Biotech Int., Rockford, Illinois, USA) according to the manufacturer's instructions.
Evaluation of Kidney Tissue
MPO Activity (Marker of Inflammation)
MPO activity was measured in tissues in a procedure similar to that documented by Hillegas et al.Citation[15] Tissue samples were homogenized in 50 mM potassium phosphate buffer (PB, pH 6.0) and centrifuged at 41,400g (10 min); pellets were suspended in 50 mM phosphate buffer containing 0.5% hexadecyltrimethylammonium bromide (HETAB). After three freeze and thaw cycles with sonication between cycles, the samples were centrifuged at 41,000 g for 10 min. Aliquots (0.3 mL) were added to 2.3 mL of reaction mixture containing 50 mM phosphate buffer, o-dianisidine, and 20 mM H2O2 solution. One unit of enzyme activity was defined as the amount of MPO present that caused a change in absorbance measured at 460 nm for 3 min. MPO activity was expressed as U/g tissue.
Biomarkers of Oxidative Stress
GSH was estimated by the Moran et al. method (1979); MDA was estimated by the Slater and Sawyer method (1971); SOD was estimated by the Misra and Fridovich method (1972); and CAT was estimated by the Hugo E. Aebi method (1983).
Tissue Nitrite Levels
The level of nitrite level was estimated by the method of Lepoivre et al.Citation[16] To 0.5 mL of tissue homogenate, 0.1 mL of sulphosalicylic acid was added and vortexed well for 30 minutes. The samples were then centrifuged at 5,000 rpm for 15 minutes. The protein-free supernatant was used for the estimation of nitrite levels. To 200 μL of the supernatant, 30 μL of 10% NaOH was added, followed by 300 μL of Tris-HCl buffer and mixed well. To this, 530 μL of Griess reagent was added and incubated in the dark for 10–15 minutes, and the absorbance was read at 540 nm against a Griess reagent blank. Sodium nitrite solution was used as the standard. The amount of nitrite present in the samples was estimated from the standard curves obtained.
Histopathology
For light microscopic evaluation, kidneys were fixed in 10% phosphate buffered formalin. Paraffin-embedded specimens were cut into 6 mm-thick sections and stained with hematoxylin and eosin (H&E). The kidneys were examined under a light microscope (Olympus Bioxl) for the presence of tubular changes and interstitial inflammatory cell infiltration by an observer blinded to the animal treatment group
DNA Fragmentation
Genomic DNA was extracted from renal cortices using DNA extraction kit (DNeasy kit, Axygen). Ten micrograms of DNA were electrophoresed on a 2% agarose gel. Fragmented DNA was visualized by ethidium bromide under an UV light source.
Statistics
All of the data are expressed as mean ± SEM. Statistical significance between more than two groups was tested using one-way ANOVA followed by the Bonferroni multiple comparisons test or unpaired two-tailed student's t-test as appropriate using a computer-based fitting program (Prism, Graphpad 5). Differences were considered to be statistically significant when p < 0.05.
RESULTS
Measurement of Systolic Blood Pressure
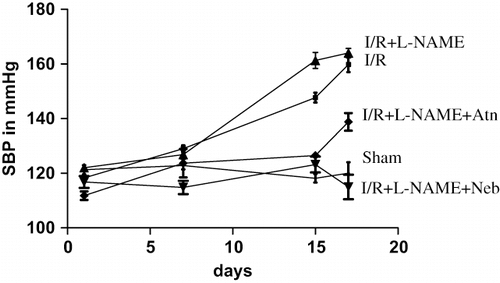
On the day of unilateral nephrectomy (day 1), the sham-operated animals showed mean SBP value 121.3 ± 1.64 mmHg, which did not significantly change up to day 17. Animals that underwent I/R showed a significant rise (p < 0.001, n = 6) in mean SBP (159.8 ± 2.78 mmHg) as compared to sham-operated group. Further high SBP (164 ± 1.67) was observed in I/R+L-NAME treated rats as compared to I/R control group alone. However, nebivolol treatment in I/R+L-NAME group had significantly (p < 0.001, n = 6) reduced the rise in the mean SBP as compared to I/R control and I/R + L-NAME group (see ).
Figure 1. Mean systolic blood pressure in rats subjected to 45-minute ischemia and 24 hours reperfusion in sham-operated, I/R control, I/R+L-NAME, I/R+L-NAME+Neb, and I/R+L-NAME+Atn groups. Unit: mmHg. Values are expressed as mean ± SEM for six animals in the group. ***p < 0.001 sham vs. I/R control and I/R+L-NAME, ***p < 0.001 I/R+L-NAME+Neb vs. I/R control and I/R+L-NAME.
Serum Estimation for Renal Function (BUN and Creatinine) and TNF-α (Marker of Inflammation)
Renal Function
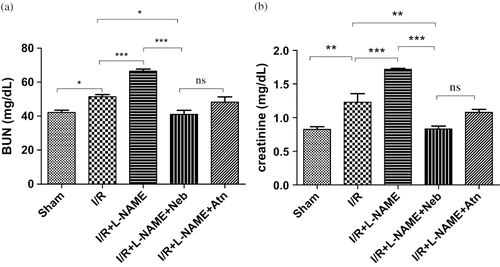
The serum creatinine and BUN were significantly increased by 48.66% (p < 0.01, n = 6) and 22.12% (p < 0.05, n = 6) in animals that underwent I/R as compared to sham-operated group (see ), indicating a significant degree of glomerular dysfunction mediated by renal I/R. In L-NAME+I/R-treated rats, serum creatinine and BUN levels were significantly (p < 0.001, n = 6) higher as compared to I/R control group alone. In the nebivolol-treated I/R+ L-NAME group, the elevated serum creatinine and BUN levels were significantly depressed as compared to I/R control (p < 0.01 and p < 0.05, n = 6) and I/R+ L-NAME (p < 0.001, n = 6) groups.
Figure 2. (a) Serum BUN levels in rats subjected to 45-minute ischemia and 24 hours reperfusion in sham-operated, I/R control, I/R+L-NAME, I/R+L-NAME+Neb, and I/R+L-NAME+Atn groups. Unit: mg/dL. Values are expressed as mean ± SEM for six animals in the group. *p < 0.05 sham vs. I/R control, ***p < 0.001 I/R control vs. I/R+L-NAME, *p < 0.05 I/R+L-NAME+Neb vs. I/R control, ***p < 0.001 I/R+L-NAME+Neb vs. I/R+L-NAME. (b) Serum creatinine levels in rats subjected to 45-minute ischemia and 24 hours reperfusion in sham-operated, I/R control, I/R+L-NAME, I/R+L-NAME+Neb, and I/R+L-NAME+Atn groups. Unit: mg/dL. Values are expressed as mean±SEM for six animals in the group. **p < 0.01 sham vs. I/R control, ***p < 0.001 I/R control vs. I/R+L-NAME, **p < 0.01 I/R+L-NAME+Neb vs. I/R control, ***p < 0.001 I/R+L-NAME+Neb vs. I/R+L-NAME.
TNF-α Quantification by ELISA
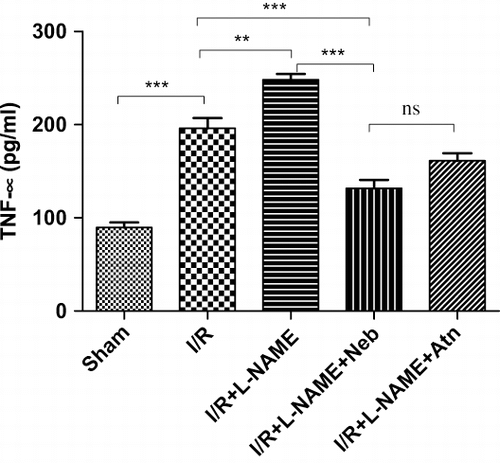
The serum TNF-α level was significantly (p < 0.001, n = 6) increased by ∼2.2-fold (196.00 ± 11.08 pg/mL) in the I/R control group as compared to sham-operated group (89.67 ± 5.38 pg/mL). In L-NAME+I/R-treated rats, serum TNF-α level was significantly (p < 0.01, n = 6) higher as compared to I/R control group alone. Treatment with nebivolol in the I/R+L-NAME group had significantly reduced serum TNF-α level (p < 0.001, n = 6) as compared to the I/R control and I/R+L-NAME groups (see ).
Figure 3. Serum TNF-α levels in rats subjected to 45-minute ischemia and 24 hours reperfusion in sham-operated, I/R control, I/R+L-NAME, I/R+L-NAME+Neb, and I/R+L-NAME+Atn groups. Unit: pg/mL. Values are expressed as mean±SEM for six animals in the group. ***p < 0.001 sham vs. I/R control, **p < 0.01 I/R control vs. I/R+L-NAME, ***p < 0.001 I/R+L-NAME+Neb vs I/R control, ***p < 0.001 I/R+L-NAME+Neb vs. I/R+L-NAME.
Evaluation of Kidney Tissue
MPO Activity
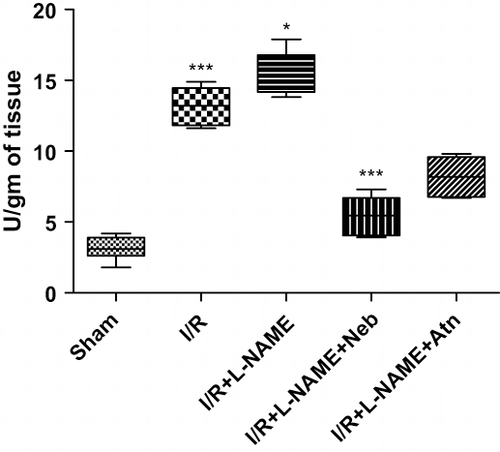
MPO activity was significantly (p < 0.001, n = 6) increased (∼5-fold) in the I/R control group as compared to sham-operated animals. In L-NAME+I/R-treated rats, MPO activity was significantly (p < 0.05, n = 6) higher as compared to I/R control group alone (see ). Treatment with nebivolol in the I/R+L-NAME group had significantly reduced MPO activity (p < 0.001, n = 6) as compared to I/R control and I/R+ L-NAME group.
Figure 4. MPO activity in kidney tissue of rats subjected to 45-minute ischemia and 24 hours reperfusion in sham-operated, I/R control, I/R+L-NAME, I/R+L-NAME+Neb, and I/R+L-NAME+Atn groups. Unit: Units/gm of tissue. Values are expressed as mean ± SEM for six animals in the group. ***p < 0.001 sham vs. I/R control, *p < 0.05 I/R control vs. I/R+L-NAME, ***p < 0.001 I/R+L-NAME+Neb vs. I/R control, ***p < 0.001 I/R+L-NAME+Neb vs. I/R+L-NAME.
Biomarkers of Oxidative Stress
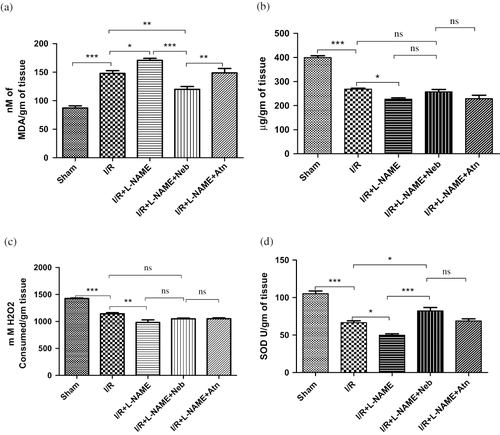
Renal I/R produced a significant increase in tissue MDA levels (from 87.0 ± 4.05 to 147.80 ± 4.71 nmol/gm of tissue, p < 0.001, n = 6) as compared to sham-operated animals. In I/R+ L-NAME-treated rats, tissue MDA level was significantly (p < 0.05, n = 6) higher as compared to the I/R control group alone. Treatment with nebivolol in I/R+ L-NAME had produced a significant reduction in MDA level (111.50 ± 6.83 nmol/gm of tissue) as compared to the I/R control (p < 0.01, n = 6) and I/R+L-NAME (p < 0.001, n = 6) groups. Renal I/R significantly decreased the antioxidant enzymatic activity of GSH (from 399.70 ± 7.72 to 268.30 ± 4.24 μg/gm of tissue, p < 0.001, n = 6), CAT (from 1426 ± 9.91 to 1141 ± 22.16 nmols of H2O2 consumed/gm of tissue, p < 0.001, n = 6), and SOD (from 105.20 ± 3.53 to 66.50 ± 2.59 U/gm of tissue, p < 0.001, n = 6) in the I/R control group as compared to the sham-operated group. In I/R+L-NAME-treated rats, tissue levels of biomarkers of oxidative stress, GSH (p < 0.05, n = 6), CAT (p < 0.01, n = 6), and SOD (p < 0.05, n = 6) were significantly reduced as compared to I/R control group alone. Treatment with nebivolol in the I/R+L-NAME group had produced a significant increase in the level of SOD as compared to I/R control (p < 0.05, n = 6) and I/R+L-NAME (p < 0.001, n = 6) group. (see ). There was no significant improvement in tissue levels of GSH and CAT in the nebivolol-treated group as compared to the I/R control and I/R+L-NAME groups.
Figure 5. (a) MDA level in kidney tissue of rats subjected to 45-minute ischemia and 24 hours reperfusion in sham-operated, I/R control, I/R+L-NAME, I/R+L-NAME+Neb, and I/R+L-NAME+Atn groups. Unit: nm of MDA/gm tissue. Values are expressed as mean ± SEM for six animals in each group. ***p < 0.001 sham vs. I/R control, *p < 0.05 I/R control vs. I/R+L-NAME, **p < 0.01 I/R+L-NAME+Neb vs. I/R control, ***p < 0.001 I/R+L-NAME+Neb vs. I/R+L-NAME. (b) GSH level in kidney tissue of rats subjected to 45-minute ischemia and 24 hours reperfusion in sham-operated, I/R control, I/R+L-NAME, I/R+L-NAME+Neb, and I/R+L-NAME+Atn groups. Unit: μg/gm of tissue. Values are expressed as mean ± SEM for six animals in each group. ***p < 0.001 sham vs. I/R control, *p < 0.05 I/R control vs. I/R+L-NAME. (c) CAT level in kidney tissue of rats subjected to 45-minute ischemia and 24 hours reperfusion in sham-operated, I/R control, I/R+L-NAME, I/R+L-NAME+Neb, and I/R+L-NAME+Atn groups. Unit: μg/gm of tissue. Values are expressed as mean ± SEM for six animals in each group. ***p < 0.001 sham vs. I/R control, **p < 0.01 I/R control vs. I/R+L-NAME. (d) SOD level in kidney tissue of rats subjected to 45-minute ischemia and 24 hours reperfusion in sham-operated, I/R control, I/R+L-NAME, I/R+L-NAME+Neb, and I/R+L-NAME+Atn groups. Unit: SOD U/gm of tissue. Values are expressed as mean ± SEM for six animals in each group. ***p < 0.001 sham vs. I/R control, *p < 0.05 I/R control vs. I/R+L-NAME, *p < 0.05 I/R+L-NAME+Neb vs. I/R control, ***p < 0.001 I/R+L-NAME+Neb vs. I/R+L-NAME.
Tissue NO Levels
Renal I/R resulted in a significant decrease in the tissue levels of nitrite (137.0 ± 2.87 nmol/gm tissue, p < 0.05, n = 6) as compared to values obtained from the tissue of sham-operated animals (158.7 ± 3.76). In I/R+L-NAME-treated rats, a significant decrease in the tissue levels of nitrite as compared to I/R control group alone (from 137.0 ± 2.87 to 112.7 ± 7.59 nmol/gm tissue, p < 0.05, n = 6). However, decreased nitrite levels mediated by renal I/R in I/R control and I/R+L-NAME were increased significantly (162.0 ± 4.53 nmol/gm tissue, p < 0.01 and p < 0.001, n = 6) after the administration of nebivolol in the I/R+L-NAME group (see ).
Table 1 Tissue nitrate level in kidney tissue of rats subjected to 45-minute ischemia and 24 hours reperfusion in sham-operated, I/R control, I/R+L-NAME, I/R+L-NAME+Neb, and I/R+L-NAME+Atn groups
Histopathology
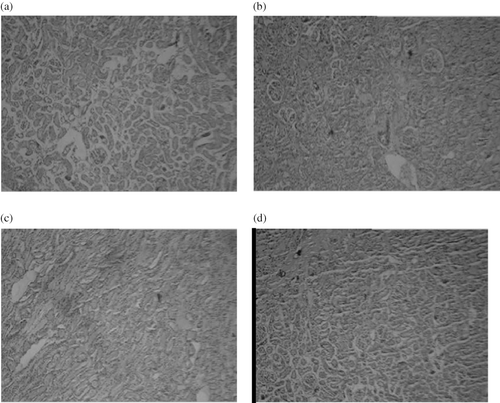
Light microscopic evaluation of the sham-operated groups revealed a regular morphology of renal parenchyma with well-designated glomeruli and tubuli (see ). In the I/R control and I/R+L-NAME groups, the interstitial hemorrhage, dilated tubuli, and prominent glomerular degeneration followed by atrophy revealed that I/R caused severe glomerular, tubular, and interstitial damage. Tubular dilation was present throughout the tissue (see ). In the nebivolol-treated I/R+ L-NAME group, there was a significant regeneration in all features of the injury. Reduced tubular dilation, loss of interstitial hemorrhage, and glomerular atrophy were the regenerated features (see ).
Figure 6. Microscopic observations of kidneys tissue sections of rats subjected to 45-minute ischemia and 24 hours reperfusion, before and after treatment with nebivolol with BIOXL light microscope showing morphological changes. Images were taken under light microscopy using hematoxylin and eosin (×10). (a) Sham, (b) I/R, (c) I/R+L-NAME, (d) I/R+L-NAME+Neb.
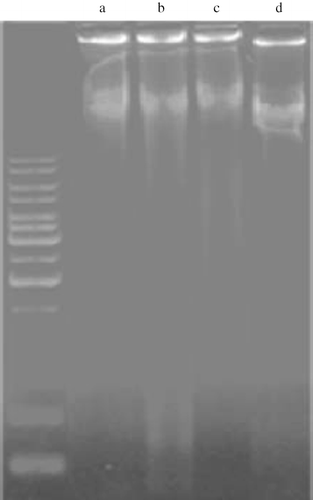
DNA Fragmentation
Apoptosis was evaluated by DNA fragmentation analysis. The typical DNA laddering activity was observed in the I/R control and in I/R+ L-NAME groups, which indicates cell necrosis. Treatment with nebivolol in I/R+ L-NAME had decreased DNA fragmentation and necrosis in comparison to the I/R control and I/R+L-NAME groups (see ).
DISCUSSION
In the clinical settings, renal I/R is a consequence of systemic hypoperfusion with subsequent circulatory resuscitation, such as following aortic cross-clamping or renal transplantation.Citation[6] There is a good evidence from both in vivo and in vitro studies that the formation of NO plays an important role in I/R.Citation[17] One of the important mechanisms for I/R injury is excessive ROS, which scavenges NO and cumulates in reduced NO bioavailability.Citation[18] The present study is undertaken to find out whether the renal injury and dysfunction produced by I/R can be reversed when the NO levels are increased by the administration of the NO donor compounds.
Previous studies have shown that iNOS inhibitors protected against I/R, which confirms the role of harmful NO in mediating cellular injuries.Citation[19],Citation[20] Moreover, the iNOS type of NOS enzyme specifically is activated during I/R. The induction of iNOS may have either toxic or protective effects, depending on the simultaneous production of oxidative stress. The generation of NO by iNOS leads to lipid peroxidation, DNA damage, and pro-apoptotic effects.Citation[19],Citation[21] Endothelial cells produce less bioactive NO in the presence of higher oxidative stress.Citation[19],Citation[22] This finding is consistent with the present study. In this study, renal I/R caused a significant increase in the renal MDA levels and depleted the antioxidant enzyme pool, as is evident from the significantly declined levels of biomarkers of oxidative stress enzymes, like SOD, CAT, and GSH, in I/R animals and the L-NAME+I/R group as compared to sham-operated animals, which indicates an increase in oxidative stress. Decreased NO levels indicate a rise in peroxynitrite concentration after I/R. Previous studies have shown that peroxynitrite level in the heart increases greater than 10-fold in the first minute of reperfusion.Citation[23] In this study, there was a significant decrease in tissue nitrite levels in kidney of I/R group animals as compared to sham-operated group. Cristol et al.Citation[24] have demonstrated the role of endothelial NO after I/R and decrease in renal function after treatment with L-NAME. Moreover, peroxynitrite could initiate lipid peroxidation, which damages the proximal tubular cells, nitration of tyrosine residues (nitrotyrosine), and nitration of cellular proteins, with a subsequent loss of protein structure resulting in reduction of the kidney function.Citation[25],Citation[27] Similar results were obtained in this study as well. There was a significant increase in the levels of serum creatinine and BUN in I/R control group, whereas animals treated with L-NAME exhibited further worsening of renal function in comparison to I/R control group.
Previous studies have shown that nebivolol concentrations were decreased after exposure to electrolysis-induced ROS, thus indicating that nebivolol is degraded by its reaction with ROS and decreases oxidative stress. Studies also revealed that NO donors' compounds can scavenge free oxygen radicals.Citation[11],Citation[28] In the present work, there was a significant reduction in the levels of MDA and increase in the levels of marker of oxidative stress like SOD in the nebivolol-treated I/R+L-NAME group as compared to the I/R control and I/R+L-NAME groups. A previous study suggested that nebivolol may induce NO production in endothelial cells via a calcium-dependent mechanism.Citation[29] There was a significant increase in tissue nitrite levels in the nebivolol-treated I/R+L-NAME group as compared to the I/R control and I/R+L-NAME groups. A reduction in oxidative stress and increase in NO level in the nebivolol-treated I/R+L-NAME group showed significant improvement in renal function as compared to the I/R control and I/R+L-NAME groups.
In the present study, there was a significant increase in mean SBP in I/R control group in comparison to the sham-operated group, and it was higher still in the I/R+L-NAME group. An important factor contributing to hypertension seems to be endothelial dysfunction, in particular the narrowing of the lumen and loss of a vasorelaxing effect caused by a reduction in NO production.Citation[30] NO deficiency increases cholinergic activity, which leads to enhance β1 adrenoreceptor activity, which ultimately stimulates renin secretion.Citation[7],Citation[31] However, previous studies have shown that β1 blockers decrease rennin release, and NO decreases vascular response to Ang II through cGMP production, respectively.Citation[32],Citation[33] In this study, there was a significant reduction in mean SBP in the nebivolol-treated I/R+L-NAME group as compared to the I/R control and I/R+L-NAME groups.
In this study, there was a significant increase in serum TNF-α level and kidney tissue MPO activity in the I/R control group as compared to the sham operated group. The inflammation involved in the progression of I/R injury may be mediated by a variety of inflammatory mediators, neutrophil infiltration, caspase activation, and the occurrence of apoptosis.Citation[34] Previous studies have shown that neutrophils can migrate to ischemic area during reperfusion period only. MPO activity is an essential parameter showing reperfusion injury in tissue.Citation[35] Previous studies have shown that NO produces anti-inflammatory action by binding with kinin β2 receptors in eNOS knockout mice.Citation[36] The present finding is similar with this hypothesis. The TNF-α level and MPO activity were significantly higher in the I/R+L-NAME-treated group as compared to the sham-operated and I/R control groups. However, NO donors can inhibit inflammatory reactions after I/R.Citation[6],Citation[37] In this study, there was a significant decrease in serum TNF-α level and tissue MPO activity in the nebivolol-treated I/R+L-NAME rats as compared to the I/R control and I/R+L-NAME groups. Thus, for the first time, this study claimed that nebivolol could produce more significant anti-inflammatory activity than any other β1 adrenoreceptor blocker.
Previous studies have shown that a major pathway of peroxynitrite-dependent cytotoxicity rely on oxidative DNA damage and activation of the nuclear enzyme poly(ADP-ribose) polymerase, leading to cell necrosis.Citation[38] In addition, in response to ROS, the outer membrane of mitochondria becomes permeabilized, resulting in the translocation of Bax from cytosol to the mitochondria and the release of cytochrome C occurs. The release of cytochrome C into the cytosol leads to form the apoptosome, which stimulates the activation of procaspase-9 and procaspase-3. Active caspase-3 activates the caspase activated DNAase, leading to DNA fragmentation.Citation[39],Citation[40] In this study, severe cell necrosis was observed in the I/R control and I/R+L-NAME-treated groups. However, previous studies have shown that NO inhibits apoptosis by S-nitrosylation of caspase-3. NO activates cGMP signaling, which causes the activation of cGK, which leads to the activation of p38 MAPK and PI3-K/Akt and the reduction of mitochondrial Ca2+ uptake and prevents apoptosis.Citation[41] In our study, decreased cell necrosis was observed in nebivolol-treated rats in comparison to the I/R- and L-NAME-treated groups.
CONCLUSIONS
In conclusion, the present findings demonstrated that nebivolol protected against warm ischemia/reperfusion-mediated renal injury in rats. We further demonstrated that nebivolol possessed both anti-apoptotic and anti-inflammatory properties apart from β1 adrenoreceptor blocker activity. These findings suggested the potential role of nebivolol against ischemia reperfusion injury during renal surgery or transplantation.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Manuela A, Juan C, Raffaella M, et al. Oxidative stress and kidney dysfunction due to ischemia/reperfusion in rat: Attenuation by dehydroepiandrosterone. Kidney Int. 2003; 64: 836–843
- Singh D, Chopra K. The effect of naringin, a bioflavonoid on ischemia-reperfusion-induced renal injury in rats. Pharmacological Research 2004; 50: 187–193
- Jan G, Reinhard S, Ulrike R, et al. l-Arginine counteracts nitric oxide deficiency and improves the recovery phase of ischemic acute renal failure in rats. Kidney Int. 2003; 64: 216–225
- Mine I, Mehmet A, Mehmet DB. The effect of quercetin on renal ischemia and reperfusion injury in the rat. Cell Biochem Funct. 2002; 20: 291–296
- Koo J, Nosratola DV. Effects of diabetes, insulin and antioxidants on NO synthase abundance and NO interaction with reactive oxygen species. Kidney Int. 2003; 63: 195–201
- Vikas C, Kanwaljit C. Renal protective effect of molsidomine and L-arginine in ischemia-reperfusion induced injury in rats. Journal of Surgical Research. 2005; 128: 132–139
- Claudio S, Mattia L, Urs S. Interaction between nitric oxide and the cholinergic and sympathetic nervous system in cardiovascular control in humans. Pharmacology & Therapeutics. 2005; 106: 209–220
- Marija MK, Jean PE, Heribert W, Richard B, Andre PS. Characterization of beta-adrenoceptor subtypes in rat kidney with new highly selective β1 blockers and their role in renin release. Biochem Pharmacol. 1985; 34: 3951–3957
- Michael P. Nebivolol: Pharmacologic profile of an ultraselective, vasodilatory 1-blocker. J Clin Pharmacol. 2008; 48: 225–239
- Pires MJ, Iguez-Peña MJ, Evalo M, et al. Long-term nebivolol administration reduces renal fibrosis and prevents endothelial dysfunc. J Hypertens 2007; 25: 2486–496
- Groot AA, Mathy MJ, Zwieten PA, Peters SL. Antioxidant activity of nebivolol in the rat aorta. J Cardiovasc Pharmacol. 2004; 43: 148–153
- Dehpour AR, Sadr SS, Nouroddini M, et al. Comparison of simultaneous administration of lithium with L-NAME or L-arginine on morphine withdrawal syndrome in mice. Human Psychopharmacology: Clinical and Experimental. 2000; 15: 87–93
- Omer T, Mustafa C, Mehmet T, et al. Preventive effect of nebivolol on contrast-induced nephropathy in rats. Nephrol Dial Transplant. 2008; 23: 853–859
- Chuan Z, Xie HH, Lu ZA, Zhu MJ, Su DF. Inhibition of inflammation contributes to organ protection of atenolol in sinoaortic-denervated rats. Journal of Cardiovascular Pharmacology 2008; 43: 663–668
- Hillegas LM, Griswold DF, Brickson B. Assessment of myeloperoxidase activity in whole rat kidney. J Pharmacol Methods 1990; 24: 852
- Lepoivre M, Chenais B, Yapo A. Alterations of ribonucleotide reductase activity following induction of nitrite-generating pathway in adenocarcinoma cells. J Biol Chem. 1990; 6: 14143–14149
- Nimesh SP, Espen OK, Salvatore C, et al. Inhibition of inducible nitric oxide synthase reduces renal ischemia/reperfusion injury. Kidney Int. 2002; 61: 862–871
- Wen HZ, Jian YL, Yong Z. Melatonin abates liver ischemia/reperfusion injury by improving the balance between nitric oxide and endothelin. Hepatobiliary Pancreat Dis Int. 2006; 5: 574–579
- Tsuchihashi S, Kaldas F, Chida N, et al. FK330, a novel inducible nitric oxide synthase inhibitor, prevents ischemia and reperfusion injury in rat liver transplantation. Am J Transplantation 2006; 6: 2013–2022
- Engin S, Hakan P, Omer FC, Yusuf T, Ahmet A. Effects of aminoguanidine against renal ischaemia-reperfusion injury in rats. Cell Biochem Funct 2004; 24: 137–141
- Michael SG, Sergey VB. Nitric oxide in acute renal failure: NOS versus NOS. Kidney Int. 2002; 61: 855–861
- Christopher SW. Reactive oxygen species: Roles in blood pressure and kidney function. Current Hypertension Reports. 2002; 4: 160–166
- Jeremy HS, Jiahui L, Brian PH, William HG. Peroxynitrite inhibits myofibrillar protein function in an in vitro assay of motility. Free Radical Biology & Medicine. 2008; 44: 14–23
- Cistol JP, Mitchell JA, Walder C, Vande JR. Support of renal blood flow after ischemia reperfusion injury by endogenous formation of nitric oxide and cycloxigenase vasodilator metabolites. Br J Pharmacol 1993; 109: 188–194
- Akira I, Yoshiaki N, Mitsutoshi T, Hitoshi H, Masao M, Kuniaki T. Sodium-dependent glucose transporter reduces peroxynitrite and cell injury caused by cisplatin in renal tubular epithelial cells. Biochimica et Biophysica Acta 2005; 1717: 109–117
- Ester WJ, Coen AS, Anton T, Adam MT, Harry G. Expression of inducible and endothelial nitric oxide synthases, formation of peroxynitrite and reactive oxygen species in human chronic renal transplant failure. American Journal of Transplantation 2002; 2: 448–453
- Lisa AM, Ann VR, James AS. Inhibition of nitric oxide synthase reduces renal ischemia/reperfusion injury. Journal of Surgical Research 2005; 129: 236–241
- Akin M, Kurukahvecioglu O, Gulbahar O, et al. Comparison of the effects of sodium nitroprusside and L-carnitine in experimental ischemia-reperfusion injury in rats. Transplantation Proceedings. 2007; 39: 2997–3001
- Carmine V, Alessandra A, Roberta P, et al. Characterization of nitric oxide release by nebivolol and its metabolites. AJH. 2006; 19: 579–586
- Frank HC, Edgaras S, Thomas H, et al. Flow- and acetylcholine-induced dilatation in small arteries from rats with renovascular hypertension—effect of tempol treatment. European Journal of Pharmacology. 2007; 566: 160–166
- Schweda F, Kurtz A. Cellular mechanism of renin release. Acta Physiol Scand. 2004; 181: 383–390
- Ali MS, Nasrin A, Radbod D. Investigation of local ACE activity and structural alterations during development of l-NAME-induced hypertension. Pharmacological Research. 2005; 52: 438–444
- Cheuk MY, Gabriel Y, Jeffrey WH, et al. Effect of beta blockade (Carvedilol or Metoprolol) on activation of the renin-angiotensin-aldosterone system and natriuretic peptides in chronic heart failure. Am J Cardiol. 2003; 92: 406–410
- Bin Y, Sunja YJ, Izabell A, et al. Inflammation and caspase activation in long-term renal ischemia/reperfusion injury and immunosuppression in rats. Kidney International. 2005; 68: 2050–2067
- Hasan E, Ersin F, Memet HE. Protection from renal ischemia reperfusion injury by an endothelin-A receptor antagonist BQ-123 in relation to nitric oxide production. Toxicology. 2006; 228: 219–228
- Hang Y, Lee C, Julie C. Nitric oxide mediates cardiac protection of tissue kallikrein by reducing inflammation and ventricular remodeling after myocardial ischemia/reperfusion. Life Sci. 2008; 82: 156–165
- Tomoaki O, Mareomi H, Go H, et al. Effect of beta-blockers on circulating levels of inflammatory and anti-inflammatory cytokines in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2001; 37: 412–417
- Sandra L, Christine VB, Benoît P, et al. Peroxynitrite is a major trigger of cardiomyocyte apoptosis in vitro and in vivo. Free Radical Biology & Medicine. 2006; 41: 886–895
- Hui C, Xiuheng L, Bingyan Z, et al. Ozone oxidative preconditioning inhibits inflammation and apoptosis in a rat model of renal ischemia/reperfusion injury. European Journal of Pharmacology. 2008; 581: 306–314
- Hyo JK, Ki WL, Mi-Sung K, Hyong JL. Piceatannol attenuates hydrogen peroxide- and peroxynitrite-induced apoptosis of PC12 cells by blocking down-regulation of Bcl-XL and activation of JNK. The Journal of Nutritional Biochemistry. 2007; 7: 459–466
- Yasuhiro M, Susumu A, Kino M, Hiroshi I, Mitsuaki I. Nitric oxide inhibits myocardial apoptosis by preventing caspase-3 activity via S-nitrosylation. Journal of Molecular and Cellular Cardiology 2005; 38: 163–174