Abstract
Aim. To establish the role of endothelin-1 and nitric oxide in the pathogenesis of hypertension in patients on chronic aemodyalisis by correlating endothelin-1 and NO plasma concentrations to the level of arterial hypertension with respect to angiotensin-converting enzyme (ACE) inhibitor therapy. Methods. We determined plasma concentrations of endothelin-1 and NO in patients on chronic hemodialysis (CHD) before and after hemodialysis treatment. The study included 30 CHD patients and 20 healthy participants as controls. Correlation to blood pressure was determined, as well as the effect of ACE inhibitors on the relationship between both endothelin-1 and NO in correlation with arterial hypertension. Main findings.Endothelin-1 plasma concentration was significantly higher in CHD patients before hemodialysis treatment than in healthy controls. Endothelin-1 plasma concentration was also significantly higher in CHD patients after hemodialysis than in healthy controls. There was a significant decrease in endothelin-1 plasma concentration after hemodialysis in comparison with its values before hemodialysis. In CHD patients, a positive correlation was found between endothelin-1 plasma concentration and systolic blood pressure after hemodialysis, irrespective of ACE inhibitors therapy. In CHD patients taking ACE inhibitors, systolic blood pressure increased with increasing endothelin-1 plasma concentration before as well as after hemodialysis. In patients taking ACE inhibitors, there was a tendency for diastolic blood pressure to increase with an increase in endothelin-1 plasma concentration after hemodialysis and to decrease with an increase in NO plasma concentration. Conclusion. NO and endothelin-1 play a significant role in etiology of the hemodynamic changes of blood pressure during the dialysis.
INTRODUCTION
Hypertension in patients on chronic hemodialysis (CHD) is a multifactorial condition. Traditionally, there are two types of hypertension in CHD patients.Citation[1],Citation[2] The first type is characterized by normal to low plasma renin activity and the possibility of decreasing high blood pressure by regulating body weight and reducing salt intake. This type of hypertension is called volume hypertension. The other type is characterized by high plasma renin activity and an inability to regulate blood pressure by reduced salt intake and normalization of body weight. This type of hypertension is called renin-dependent hypertension.Citation[3],Citation[4] Recently, increased consideration has been given to the influence of endothelin-1 and nitric oxide (NO) in the pathogenesis of hypertension in CHD patients. Vascular endothelium plays a central role in the regulation of vascular tone via production of vasoactive substances, including endothelin-1 as a vasoconstrictor and NO as a vasodilator. Endothelin-1 increases the activity of sympathetic nervous system and is the most powerful vasoconstricting substance known so far.Citation[5–11] It increases arterial blood pressure, reduces renal plasma flow and glomerular filtration, and decreases natriuresis and diuresis.Citation[12–15] In the glomeruli affected by sclerosis, endothelial injury leads to the increased secretion of endothelin-1 and consequent vasoconstriction, increased intraglomerular pressure, and decreased glomerular filtration. NO is a free radical involved in various physiological processes. NO leads to vasodilatation and inhibits the proliferation of vascular smooth muscle as well as the adhesion and aggregation of blood platelets. Produced by NO synthase from substrate L-arginine in vascular endothelial cells, NO acts as a potent vasodilator contributing to the regulation of vascular tone.Citation[16–18] In the uremic condition, endothelial dysfunction and reduced production and effect of NO have been observed.
Decreased NO levels are the result of the lack of L-arginine due to the loss of functional renal mass.Citation[19–21] Increased levels of endogenous NO synthase inhibitors, such as asymmetric dimethylarginine (ADMA), are also found.Citation[19],Citation[22–24] Circulating levels of NO synthase inhibitors accumulate in terminal stages of renal failure, when changes in the blood pressure in patients on hemodialysis are also observed.Citation[25],Citation[26] In patients with renal failure, the concentration of L-arginine analogues increases. L-arginine analogues competitively inhibit NO synthase, decrease L-arginine plasma concentration, and may induce arterial hypertension. They also lead to immune system damage due to impairment of NO production in the endothelium and macrophages. When renal mass in glomeruli is reduced, inflammatory mediators are increasingly secreted as well as platelet derived growth factor (PDGF) and transforming growth factor β (TGF-β). PDGF and TGF-β are potent inhibitors of NO synthase, and they block interleukin-1B-induced mRNA in a dose-dependent manner for inducible NO synthase in mesangial cells.Citation[25],Citation[26] The most recent results of in vitro and in vivo studies on rats have shown that reduced renal mass leads to an increased synthesis of a potent vasoconstrictor, endothelin-1, which then reduces the production of NO.Citation[27],Citation[28]
The aim of our study was to determine and compare plasma concentration of endothelin-1 and NO in CHD patients before and after hemodialysis treatment with those in healthy controls, and to correlate endothelin-1 and NO plasma concentrations to the level of arterial hypertension with respect to angiotensin-converting enzyme (ACE) inhibitor therapy.
MATERIALS AND METHODS
The study included 30 CHD patients, 18 men and 12 women (age range: 50–80 years; mean: 65) with various primary diseases (see ). Half of the CHD patients were taking ACE inhibitors for the treatment of hypertension, whereas the other half did not take these medications. The control group included 20 healthy participants (12 men and 8 women) aged 63 years on average. Inclusion criteria were the absence of known kidney disease, hypertension, heart disease, diabetes mellitus, and systemic or malignant disease. There was no significant difference in the age and sex composition between the patient and control group. All study participants underwent a clinical examination and kidney ultrasound examination (renal size). Hemoglobin and creatinine serum concentration were also determined. Endothelin-1 and NO were measured before and after hemodialysis. In all patients, the dose of erythropoetin (see ) and the type of dialysis membrane were determined. Half of them used Fresenius F6, and the other half used Fresenius F7 membrane. The data on ACE therapy and concomitant antihypertensive therapy were collected from patient medical records (see ). The nature of the study was explained, and all subjects gave written consent to participate. The approval from Ethics Committe of Mostar University School of Medicine was obtained.
Table 1 Chronic hemodialysis patients regarding primary disease
Table 2 Chronic hemodialysis patients regarding erythropoetin dose
Table 3 Chronic hemodialysis patients regarding ACE inhibitors and concomitant antihypertensive therapy
Endothelin-1 was determined by enzyme-linked immunosorbent assay (ELISA). Endothelin test kit used in this study was an enzyme immunoassay designed for direct determination of endothelin in biological fluids (Endothelin ELISA-Enzyme-Linked Immunoasorbent Assay, The Next Generation, Cat. No. BI-20052, 12 × 8 test, Biomedica Gruppe, Wien, Austria).Citation[7] NO was determined from NO2, a stable product of NO, using Griess reaction and colorimetric determination of the concentration of colored product. Nitrites in urine were also determined with Griess reagent. Nitrite concentration was determined from the absorbance at 540 nm in the reagent solution.Citation[29]
Statistical Analysis
Normal distribution of data was determined with Kolmogorov-Smirnov goodness-of-fit test. The significance of differences in interval variables was tested with Mann-Whitney non-parametric test if two groups of data were independent, whereas Wilcoxon non-parametric test was applied to compare dependent samples. Correlation between two interval variables or an interval and binary variable was determined by Spearman non-parametric correlation coefficient (rs); significance of correlation was also calculated.
A p values < 0.05 was considered significant. Values were expressed as means +/− SD.
RESULTS
Plasma concentration of endothelin-1 was significantly higher in patients before hemodialysis than in healthy controls (17.02 pg/mL vs. 8.10 pg/mL, respectively; p < 0.001). In addition, plasma concentration of endothelin-1 was significantly higher in patients after hemodialysis (13.53 pg/mL) than in healthy controls (p < 0.001). There was a significant decrease in endothelin-1 plasma concentration in CHD patients after hemodialysis treatment in comparison with its values before hemodialysis (p = 0.001). NO plasma concentration in CHD patients before hemodialysis treatment was 5.66 μmol/L (i.e., not significantly different from NO plasma concentration of 5.88 μmol/L in healthy controls; p = 0.630). The average value of blood pressure in CHD patients and healthy controls was 142/82 mm Hg and 122/81 mm Hg, respectively (see ).
Table 4 Plasma endothelin-1 (ET-1) and nitric oxide (NO) concentrations in patients on chronic hemodialysis (CHD) before and after dialysis and healthy controls
In all patients (i.e., those taking or not taking ACE inhibitors), a positive correlation was found between endothelin-1 plasma concentration and systolic blood pressure after hemodialysis (see ). There was an obvious tendency for systolic blood pressure to increase with an increase in endothelin-1 plasma concentration after hemodialysis treatment, as shown by the squared regression coefficient of R2 = 0.1062 in linear regression analysis. Spearman's non-parametric correlation showed a statistically significant, positive correlation (rs = 0.38; p = 0.030).
Figure 1. Correlation between systolic blood pressure and plasma concentration of endothelin-1 after hemodialysis in all patients irrespective of ACE inhibitor therapy.
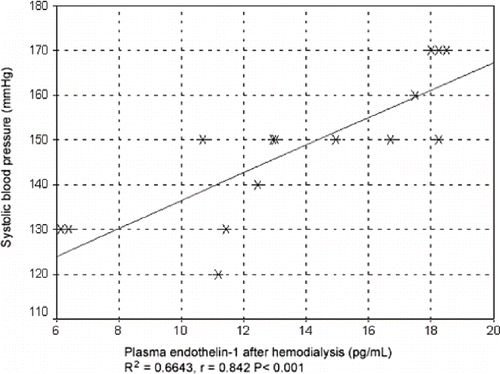
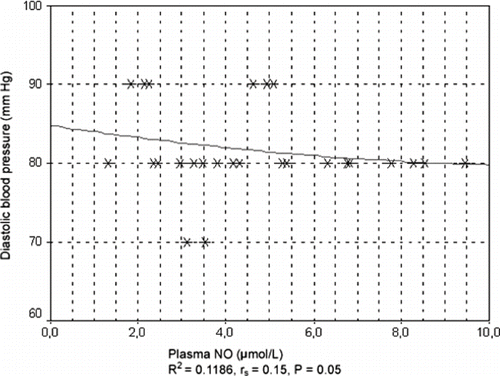
Before hemodialysis, systolic blood pressure in patients taking ACE inhibitors increased with an increase in endothelin-1 plasma concentration. A linear model had a squared regression coefficient value of R2 = 0.1063. Spearman's non-parametric correlation coefficient showed a statistically significant positive correlation (rs = 0.28, p = 0.310). After hemodialysis, there was a tendency for systolic blood pressure in patients taking ACE inhibitors to increase with an increase in endothelin-1 plasma concentration (see ). In these patients, correlation regression analysis showed a pronounced tendency for systolic blood pressure to increase when endothelin-1 plasma concentration reached high values. There was a positive regression trend, following a linear model indicated by a high value of squared regression coefficient, R2 = 0.06643, in these patients. Spearman's correlation coefficient was rs = 0.842 (p < 0.001; see ). In patients taking ACE inhibitors, there was a tendency for diastolic blood pressure to increase with increasing endothelin-1 plasma concentration after hemodialysis, as shown by a regression coefficient of R2 = 0.3343 for squared model. Correlation and regression analysis showed a tendency for systolic blood pressure values to decrease with an increase in NO plasma concentration before hemodialysis in patients taking ACE inhibitors, with squared regression coefficient of R2 = 0.0961 for squared model indicating a negative correlation. Spearman's correlation coefficient was rs= −0,271 (P = 0.140). Correlation and regression analysis showed a slight tendency for diastolic blood pressure values to decrease with an increase in NO plasma concentration (rs = 0.15, p = 0.05), which was also indicated by regression coefficient of R2 = 0.1186 for squared model (see ).
Figure 2. Correlation between systolic blood pressure and plasma concentration of endothelin-1 after hemodialysis in patients taking ACE inhibitors.
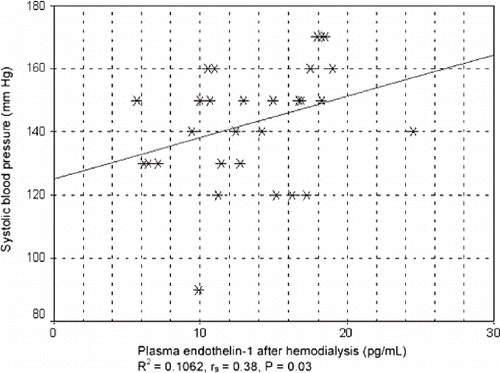
Figure 3. Correlation between diastolic blood pressure and plasma concentration of NO before hemodialysis in all patients irrespective of ACE inhibitor therapy.
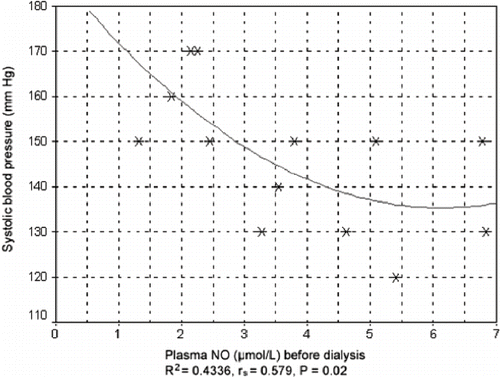
In patients taking ACE inhibitors, correlation and regression analysis showed a statistically significant decrease in systolic blood pressure before hemodialysis, which correlated with the increase in NO plasma concentration (Spearman's rs = 0.579, p = 0.020). The value of squared regression coefficient for a squared model was R2 = 0.4336 (see ).
Figure 4. Correlation between systolic blood pressure and plasma concentration of NO before hemodialysis in patients taking ACE inhibitors.
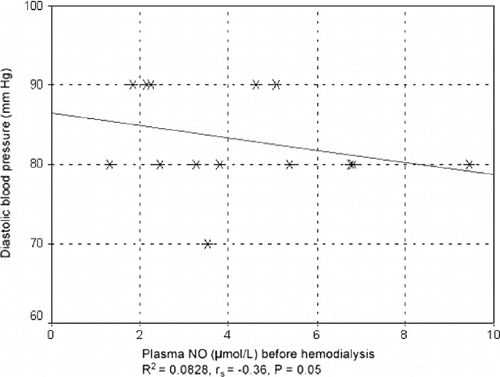
In patients taking ACE, correlation and regression analysis indicated a slight tendency for diastolic blood pressure to decrease before hemodialysis as NO plasma concentration increased (rs = -0.36; p = 0.050). This was also shown by the regression coefficient value of R2 = 0.0828 for a linear model (see ).
Figure 5. Correlation between diastolic blood pressure and plasma concentration of NO before hemodialysis in patients taking ACE inhibitors.
No significant correlation was found betwen blood pressure, endothelin-1 levels, or NO levels regarding the erythropoetin therapy.
DISCUSSION
We investigated the correlation between arterial hypertension and endothelin-1 plasma concentration in CHD patients before and after hemodialysis. We found that endothelin-1 plasma concentration was higher in CHD patients than in healthy controls. A decrease in endothelin-1 plasma concentration after hemodialysis was also observed. These results are in accordance with the findings of other authors, who observed that endothelin-1 plasma concentration and big ET-1 were increasing with the progression of renal failure.Citation[5],Citation[30]
In our study, the patients were divided in two groups, one consisting of patients taking ACE inhibitors and the other consisting of patients not taking ACE inhibitors. In patients taking ACE inhibitors, we noticed a positive correlation between endothelin-1 plasma concentration and systolic blood pressure values before and after hemodialysis. However, in patients not taking ACE inhibitors, there was a negative correlation between endothelin-1 plasma concentration and systolic blood pressure. In patients taking ACE inhibitors, we observed a positive correlation between endothelin-1 plasma concentration and diastolic blood pressure. Although we did not include renin-angiotensin system and the effects of ultrafiltration in this study, our results support the idea that ET-1 could influence on blood pressure in hemodialysis patients.
In glomeruli affected by sclerosis, damage to the endothelium leads to an increased secretion of endothelin-1, consequent vasoconstriction, increase in intraglomerular pressure, and decrease in glomerular filtration. The most recent in vitro and in vivo data on rats have shown that a decreased renal mass leads to an increased synthesis of a potent vasoconstrictor, endothelin-1, which decreases the production of NO.Citation[27],Citation[28]
The endothelins are powerful vasoconstrictor peptides of which endothelin-1 is the major isoform. Endothelin-A (ETA) and endothelin-B (ETB) receptors are found in vascular smooth muscle, where their activation modulates vasoconstriction. In one study, Goddard et al. have shown that endothelin receptor antagonists used in patients with chronic renal failure decreased blood pressure.Citation[31] They conducted a randomized, placebo-controlled, double-blind study comparing ET receptor antagonists of ETA and ETB, given alone and in combination, in eight hypertensive chronic renal failure patients and eight matched healthy controls. ETA antagonist, alone and in combination with ETB antagonist, reduced blood pressure in chronic renal failure versus placebo.
Ottosson-Seeberger et al. investigated a relationship between blood pressure and endothelin-1 plasma concentration, but found no statistically significant correlation. They observed a significant decrease in metabolic degradation of endothelin-1 in the uremic condition and found that the half-life of exogenous endothelin-1 was increased in hemodialyzed patients. However, they found no correlation between endothelin-1 concentration in subendothelial space and its plasma concentration in peripheral veins.Citation[32] Hand et al. conducted a functional investigation of correlation between endothelin-1 and blood pressure in CHD patients and found a decreased influence of endothelin-1 on the vascular tone.Citation[33]
Demudh et al., who measured endothelin-1 plasma concentration and monitored cardiovascular changes in CHD patients, reported a significant correlation between endothelin-1 plasma concentration and cardiovascular events.Citation[34] Similar results were obtained by Nabokov et al., who investigated atherosclerosis and renal failure on a rat model and found a correlation between endothelin-1 plasma concentration and atherosclerotic changes.Citation[35]
Vascular endothelium plays an important role in the tone of blood vessels and atherosclerosis prevention. In the uremic condition, endothelial dysfunction and decreased production and activity of NO was found.Citation[36],Citation[37] A decreased NO concentration was the result of the lack of L-arginine due to the loss of functional renal mass.Citation[19–21] An increased concentration of endogenous NO synthase inhibitors, such as ADMA, was also observed.Citation[19],Citation[20],Citation[22–24] Circulating levels of NO synthase inhibitors accumulate in the terminal stages of renal failure, and the blood pressure in patients on hemodialysis changes.Citation[25],Citation[26]
Haynes et al. have shown that the systemic administration of nitric oxide synthase inhibitor NG-mono-methyl-L-arginine (L-NMMA) to humans causes vasoconstriction and increases arterial pressure, confirming that nitric oxide is generated physiologically to oppose vasoconstriction influences in resistance vessels. In this study, eight healthy men received an inhibitor of nitric oxide synthase (L-NMMA) and saline placebo intravenously. L-NMMA significantly increased mean arterial pressure and total peripheral resistance. L-NMMA also significantly decreased plasma nitrate and urinary excretion of nitrate.Citation[38]
In patients with renal failure, the increased concentration L-arginine analogues, which competitively inhibit NO synthase, decreases the plasma level of L-arginine and may induce arterial hypertension. When renal mass in the glomeruli is decreased, inflammatory mediators, PDGF, and TGF-β are secreted in large amounts. PDGF and TGF-β are potent inhibitors of NO synthase and block in a dose-dependent manner IL-1B-induced mRNA for inducible NO synthase in mesangial cells.Citation[25],Citation[26]
NO deficiency has been reported in children and adults with chronic renal disease.Citation[39] In their study, Schmidt et al. have shown that total NO production was low in patients with chronic renal failure, and they concluded that this NO deficiency may contribute to the increased blood pressure that occurs in end stage renal disease. These authors have discussed the causes of deficiency in total NO production in chronic renal failure and concluded that the lack of functional renal mass will compromise the main endogenous sources of arginine generation and could lead to arginine deficiency and impaired NO synthesis.Citation[40] There is also evidence that circulating levels of endogenous NO synthase inhibitors increase in renal failure. Of these NO synthase inhibitors, ADMA acts as a potent nonselective NO synthase inhibitor, whereas symmetric dimethylarginine (SDMA) is ineffective.Citation[41]
In several animal models of renal disease, the administration of L-arginine decreased the degree of glomerulosclerosis, infiltration of macrophages, and tubulointerstitial changes, probably due to increased NO synthesis. L-arginine/NO pathways play an important role in hypertension, renal disease, and atherosclerosis. These pathways interact with renal renin-angiotensin system, endothelins, cytokines, and nuclear factor kappa B(NF-κB).Citation[42]
In rats with subototal nephrectomy, 24-hour NO excretion is reduced,Citation[43–45] suggesting reduced total NO production. Reduced urinary NO excretion has also been reported in children with chronic renal disease in adults with IgA nephropathy and hypertension and in adults with chronic renal disease by a variety of causes.Citation[23],Citation[39],Citation[46]
In our study, CHD patients did not have lower NO plasma concentration than did healthy controls. We noticed a negative correlation between NO plasma concentration and systolic blood pressure. In CHD patients before hemodialysis, a negative correlation was observed between NO plasma concentration and diastolic blood pressure. In CHD patients taking ACE inhibitors, a negative correlation between NO plasma concentration and diastolic blood pressure was found both before and after hemodialysis. Although endotelin-1 concentration was lower after dialysis, the concentration of NO did not change after dialysis. One possible explanation for the difference between NO and endothelin-1 could be the use of ACE inhibitors in the treatment of hypertension, as ACE inhibitors could increase NO level.
NO metabolism is altered in patients on hemodialysis. Circulating concentrations of NO synthase inhibitors accumulate in terminal stages of renal failure and blood pressure during hemodialysis changes. These changes in blood pressure correlate with NO concentration. Higher NO concentrations appear with a decrease in blood pressure, whereas lower NO concentrations appear with an increase in blood pressure during hemodialysis.Citation[19],Citation[20] In patients on hemodialysis, NO plasma concentration was found to be high (due to a complete loss of renal clearance), although the total NO production was low.Citation[47] CHD patients known to suffer from atherosclerosis have higher concentrations of ADMA in comparison with patients on hemodialysis without verified atherosclerosis.Citation[48]
In conclusion, the etiology of dialysis-induced hypertension remains speculative. There is mounting evidence that nitric oxide (NO) and endothelin (ET-1) may play a vital role in etiology of the hemodynamic changes of blood pressure during the dialysis. Our results, as well as those of other investigators, have shown that nitric oxide and endothelin balance may be involved in the pathogenesis of dialysis hypertension. Further studies will be required in order to definitely ascertain the role of endothelin-1 and nitric oxide in the pathogenesis of hypertension in chronic renal failure.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- de Leeuw PW. Patophysiology of hypertension in patients in renal replacement therapy. Blood Purif. 1994; 12: 245–251
- Davies DL, Schalekamp MA, Beevers DG, et al. Abnormal relation between exchangeable sodium and the renin-angiotensin system in malignant hypertension and in hypertension with chronic renal failure. Lancet. 1973; 1: 683–686
- Campigano JL, Ramirez-Muzo O, Ramirez-Gonzales R. Normal renin uremic hypertension: Study of cardiac hemodynamics, plasma volume, extracellular fluid volume, and the renin- angiotensin system. Arch Intern Med. 1976; 136: 17–23
- Tuckman J, Benninger JL, Reubi F. Hemodynamics and blood volume studies in long-term haemodialysis patients and in patients with successfully transplanted kidneys. Clin Sci Mod Med. 1973; 45: 155–157
- Pollock D. Renal endothelin in hypertension. Curr Opin Nephrol Hypertension. 2000; 9: 157–164
- Stjerniguist M. Endothelins—vasoactive peptides and growth factors. Cell Tissue. 1998; 292: 1–9
- Rossi GP, Seccia TM, Albertin G, Pessina C. Mesaurment of endothelin: Clinical and research use. Ann Clin Biochem. 2000; 37: 608–626
- Benatti L, Fabbrini MS, Patrono C. Regulation of endothelin-1 biosynthesis. Ann NY Acad Sci. 1994; 714: 109–121
- Hickey KA, Rubanyi GM, Paul RJ, Highsmith RF. Characteraziation of a coronary vasoconstrictor peptide produced by cultured endothelial cells. Am J Physiol. 1985; 248: 550–556
- Yanagisawa M, Kurihara H, Kimura S, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1998; 332: 411–415
- Shiffrin EL. Endothelin in hypertension. Curr Op Cardiol. 1995; 10: 485–494
- Simonson MS, Dunn MJ. Renal actions of endothelin peptides. Curr Opin Nephrol Hypertens. 1993; 2: 51–60
- Bloom IZ, Bentlen FR, Wilson MA, et al. In vivo effects of endothelin on the renal microcirculation. J Surg Res. 1993; 54: 274–280
- Rabelnik TJ, Kaasjager KAH, Boer P, et al. Effects of endothelin 1 on renal function in humans. Implications for physiology and patophysiology. Kidney Int. 1994; 46: 376–381
- Mongensen CE, Keeane WF, Bennett PH, et al. Prevention of diabetic renal disease with special reference to microalbuminuria. Lancet. 1995; 346: 1080–1084
- Morris ST, McMurray JJ, Spiers A, Jardine AG. Impaired endothelial function in isolated human uremic resistance arteries. Kidney Int 2001; 60: 1077–1082
- Rodrigez-Manas L, Lopez-Doriga P, Petider R, et al. Effect of glycemic control on the vascular nitric oxide system in patients with type 1 diabetes. J Hypertension. 2003; 21: 1137–1143
- Williams SB, Cusco JA, Roddy M-A, Johnstone MT, Creager MA. Impaired nitric oxide- mediated vasodilatation in patients with non-insulin dependent diabetes mellitus. J Am Coll Cardiol. 1996; 27: 567–574
- Reyes AA, Karl IE, Klahr S. Role of arginine in health and in renal disease. Am J Physiol. 1994; 267: 331–346
- Vallance P, Leone A, Calver A, Collier J, Moncada S. Acumulation of an endogenus inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992; 339: 572–575
- Schmidt RJ, Baylis C. Total nitric oxide production is low in patients with chronic renal disease. Kidney Int 2000; 58: 1261–1266
- Hand MF, Haynes WG, Webb DJ. Hemodialysis and L arginin but not D arginin correct renal faliure associated endothelial dysfunction. Kidney Int. 1998; 53: 1068–1077
- Kari JA, Donald AE, Vallance DT, et al. Physiology and biochemistry of endothelial function in children with chronic renal failure. Kidney Int. 1997; 52: 468–472
- Morris ST, McMurray J, Rodger R, Jardine AG. Impaired endothelinum-dependent vazodilatation in uremia. Nephron Dial Transplant. 2000; 15: 572–575
- Vallance P, Leone A, Calver A, et al. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet 1992; 339: 572–575
- Madore F, Prudhomme L, Austin JS, et al. Impact nitric oxide in blood pressure in hemodialysis patients. Am Kidney Dis. 1997; 30: 665–671
- Potter GS, Johnson RJ, Fink GD. Role of endothelin in hypertension of experimental chronic renal failure. Hypertension 1997; 1578–1584
- Bussemaker E, Passauer J, Reimann D, Schulze B, Reichel W, Gross P. The vascular endothelin system is not overactive in normotensive hemodialysis patients. Kidney Int. 2002; 62: 940–948
- Nims RW, Darbyshire JF, Saavedra JE, et al. Colorimetric methods for the determination of nitric oxide concentration in natural aqueous solutions. Methods 1995; 7: 48–54
- Raj DSC, Vincent B, Simpson K, et al. Hemodynamic changes during hemodylisis: Role of nitric oxide and endothelin. Kidney Int. 2002; 61: 697–704
- Goddard J, Johnston NR, Hand MF, et al. Endothelin-a receptor antagonism reduces blood pressure and increases renal blood flow in hypertensive patients with chronic renal failure. Circulation. 2004; 109: 1186–1193
- Ottosson-Seeberger A, Alhorbg G, Hemsen A, et al. Hemodialysis effects of endothelin-1 and big endothelin-1 in chronic hemodialysis patients. J Am Soc Nephrol. 1999; 10: 1037–1044
- Hand MF, Haynes WG, Webb DJ. Reduced endogenous endothelin-1 mediated vascular tone in chronic renal failure. Kidney Int. 1999; 55: 613–620
- Demuth K, Blacher J, Guerin AP, et al. Endothelin and cardiovascular remodelling in end- stage renal disease. Nephrol Dial Transplant. 1998; 13: 375–383
- Nabokov AV, Smann K, Wessels A, et al. Endothelin receptor antagonists influence cardiovascular morphology in uremic rats. Kidney Int. 1999; 55: 512–519
- Morris ST, McMurray JJ, Rodger R, Jardine AG. Impaired endothelium-dependent vasodilatationem in uremia. Nephrol Dial Transplant 2000; 15: 572–575
- Ross R. The patogenesis of atherosclerosis. A perspective for the 1990. Nature. 1993; 362: 801–809
- Haynes WG, Hand MF, Dockrell MEC, et al. Physioogical role of nitric oxide in regulation of renal function in humans. Am J Physiol. 1997; 272: 364–371
- Blum M, Yachnin T, Wollman Y, et al. Low nitric oxide production in patients with chronic renal failure. Nephron. 1998; 79: 265–268
- Schmidt RJ, Yokota S, Tracy TS, Sorkin MI, Baylis C. Nitric oxide production is low in end-stage renal disease patients on peritoneal dialysis. Am J Phisiol Renal Phisiol 1999; 276: 794–797
- Vallance PA, Leone A, Calver J, Collier J, Moncada S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet 1992; 339: 572–575
- Klahr S. The role of nitric oxide in hypertension and renal disease progression. Nephrol Dial Transplant. 2001; 16: 60–62
- Ashab I, Peer G, Blum M, et al. Oral administration of L-arginine and captopril in rats prevents chronic renal failure by nitric oxide production. Kidney Int. 1995; 47: 1515–1521
- Aiello S, Noris M, Todeschini M, et al. Renal and systematic nitric ocxide synthesis in rats with renal mass reduction. Kidney Int. 1997; 52: 171–181
- Vaziri ND, Ni Z, Wang XQ, Ovejsi F, Zhou XI. Downregulation of nitric oxide synthesis in chronic renal insufficiency: Role of excess PTH. Am J Physiol. 1998; 274: 642–649
- Kovac T, Barta J, Kossis B, Nagy J. Nitric oxide in IgA nephropathy patients with or without hypertension. Exp Nephrol. 1005; 3: 369–372
- Schmidt RJ, Domico J, Samsel LJ, et al. Indices of activity of the nitric oxide system in patients on hemodialysis. Am J Kidney 1999; 34: 228–234
- Kielstein JT, Boger RH, Bode-Boger SM, et al. Asymmetric dimetylarginine plasma concentration differ in patients with end stage renal disease: Relationship to treatment method and atherosclerosis disease. J Am Soc Nephrol. 1999; 10: 594–600