Abstract
Background. Hearing loss is common in patients with CRF. The exact etiology of this complication is not known, and little can be done to ameliorate the disorder. ADMA is found to be high in CRF patients. We studied the relation between ADMA and hearing loss in patients with CRF under conservative treatment. Methods. The study was carried out on 40 patients with renal impairment under conservative treatment (group 1) and 30 normal control subjects (group 2). For both groups' medical history and examination, biochemical tests, otological examination, pure tone audiometry, high sensitivity CRP, and asymmetric dimethylarginine (ADMA) were completed. Results. High-frequency hearing impairment was the predominant auditory dysfunction in CRF patients who showed worse high-tone hearing level on pure tone audiometry as compared with the controls (p < .001). Multiple regression analysis for hearing level at high frequency in group 1 shows that significant determinants of hearing level are ADMA (p = 0.002), high sensitivity CRP (p = 0.02), duration of renal disease (p = 0.01), diabetes mellitus (p < 0.001), and serum creatinine (p = 0.008). No correlation was found between hearing loss with age, gender, smoking, hematocrit, or lipid parameters. Conclusion. Patients with CRF under conservative treatment often experience a significant frequency hearing loss. Such a hearing disorder is mainly affected by duration and degree of renal disease, presence of DM, and level of hsCRP and ADMA. There is a close correlation between ADMA and hearing loss. Thus, ADMA could be an important factor causing hearing loss in those patients. Modifying this factor can be of value to ameliorate this common complication.
INTRODUCTION
Sensorineural hearing loss is frequently reported in patients with chronic renal failure. Several etiological factors have been linked to hearing loss in renal failure, including the use of ototoxic medications, electrolyte disturbances, hypertension,Citation[1] vitamin D deficiency,Citation[2] serum creatinine levels, Na+, K+-activated ATPase,Citation[3] and decreased conduction velocity in sensory and motor units.Citation[4]
Asymmetric dimethylarginine (ADMA) is a naturally occurring amino acid,Citation[5] which is mainly eliminated by enzyme degradation by dimethylarginine dimethylaminohydrolase (DDAH).Citation[6]
ADMA is an endogenous inhibitor of NO synthesis. In the blood vessel, NO relaxes vascular smooth muscle to increase blood flow, and suppresses processes involved in vascular disease, including leukocyte adhesion, platelet aggregation, and vascular smooth muscle cell proliferation. NO is also important in vascular regeneration as it mediates angiogenesis and mobilizes circulating endothelial progenitor cells.Citation[7]
An increase in serum ADMA levels is often observed in subjects with hypercholesterolemia, insulin resistance, diabetes mellitus, hypertension, and chronic renal disease. These conditions are associated with vascular oxidative stress, which is known to impair DDAH activity. ADMA is considered a uremic toxin, which mediates many cardiovascular complications in uremic patients.Citation[8]
The accumulation of ADMA may lead to impaired nitric oxide (NO) synthesis, and contributes to the hypertension and immune dysfunction associated with chronic renal failure.Citation[9] There are substantial data pointing out the elevation in levels of ADMA and oxidative stress markers in patients with CKD.Citation[10]
ADMA is not only a uremic toxin, but also a strong marker of endothelial dysfunction and atherosclerosis and a solid predictor of mortality in selected patient populations. It is considered a common pathway mediating the adverse vascular effects.Citation[11] We studied sensorineural hearing loss in patients with renal impairment and the possible relation to inflammation and ADMA.
PATIENTS AND METHODS
The study was carried out on 40 patients with chronic renal insufficiency under conservative treatment (group 1) and 30 normal control subjects (group 2). Inclusion criteria are patients with CRF under conservative treatment, and age- and sex-matched normal controls.
For both groups the following was completed:
Complete history and physical examination, including neurological examination. The duration of renal disease was considered from the first diagnosis of renal disease.
Otological examination was carried out, and hearing assessment using pure tone audiometry was performed.
Biochemical tests, including CBC, hematocrit, lipid profile (total cholesterol, LDL, HDL, triglyceride), RBS, serum creatinine, and BUN.
High sensitivity CRP (hsCRP).
Asymmetric dimethylarginine (ADMA).
Exclusion criteria for all included the presence of Alport's disease or other hereditary or congenital syndromes involving both the ear and the kidney, the presence of ototoxic/amino glycoside-induced hearing loss, and other known causes of hearing loss (active or recent history of otological disease, ear surgery, head injury or exposure to excessive noise).
Antihypertensive drugs used by patients included calcium channel blockers (32 patients), angiotensin-converting enzyme inhibitors (23 patients), alpha blockers (14 patients), and beta blockers (12 patients).
An otological examination was carried out to exclude middle ear pathology and conductive hearing loss. All patients had normal hearing at otoscopy, normal tuning fork tests, impedance and stapedial reflexes, and no air-bone gap on audiometric testing.
Pure tone audiometry was performed on each patient in a soundproof room by a trained audiological technician on a calibrated diagnostic audiometer, thus ensuring test-retest reliability. Change in weight of the patients was documented as an indication of volume depletion. Where a sensorineural hearing loss was shown, loudness discomfort levels and intact stapedial reflexes indicated a hearing loss of cochlear origin. The results were documented as low (125 and 250 Hz), middle (0.5, 1, and 2 kHz), and high (4 and 8 kHz) frequency losses. A threshold shift of 30 dB at one or more frequencies in each bandwidth was recorded.
Plasma total cholesterol, triglyceride, and HDL cholesterol were determined by standard enzymatic procedures. LDL cholesterol levels were calculated in subjects with triglyceride levels <400 mg/dL using the formula of Friedewald et al.Citation[12] Hematocrit and biochemistry were determined by routine techniques using an automated analyzer.
High sensitivity CRP levels were measured using an enzyme-linked immunoabsorbent assay, standardized according to the World Health Organization First International Reference Standard.Citation[13]
MEASUREMENT OF ADMA
The measurement of ADMA was accomplished by means of high-performance liquid chromatography. 1 mL of serum, to which 20 mg of 5-sulfosalicylic acid was added, and the mixture was left in an ice bath for 10 minutes. Precipitated protein was removed by centrifugation at 2,000 g for 10 minutes. Ten microliters of supernatant, which was filtered through a 0.2 μm filter, was mixed with 100 μL of derivatization reagent (prepared by dissolving 10 mg of o-phtaldialdehyde in 0.5 mL of methanol, 2 mL of 0.4 mol/L of borate buffer [pH 10.0], and 30 μL of 2-mercaptoethanol) and injected into the chromatographic system. Separation of ADMA was achieved with a 150 mm × 4 mm internal diameter. A Nova-pak C18 column with a particle size of 5 μm (Waters, Millipore, Milford, Massachusetts, USA) using 50 mmol/L of sodium acetate (pH 6.8), methanol, and tetrahydrofuran as mobile phase (A, 82:17:1; B, 22:77:1) at a flow rate of 1.0 mL/min were used. Areas of peaks detected by a fluorescent detector (excitation, 338 nm; emission, 425 nm) were used for the quantification of ADMA levels in serum. Variability of the method was less than 7%, and detection limit of the assay was 0.1 μmol/L.Citation[14]
Statistical Analysis
The calculations were performed using SPSS software program version 15. Data are expressed as means ± SD; nominal variables are expressed in frequency. Student's t test was used to compare levels between patients and controls. Correlations were tested by multiple regression analysis. Not normally distributed variables were log-transformed before entering regression analysis. A p value <0.05 is considered statistically significant.
RESULTS
Group one includes 40 patients with renal impairment with 18 females (45%) and 22 males (55%), while the control group includes 11 females (36.7%) and 19 males (63.3%). The two groups are comparable regarding age (48.8 ± 7.2 and 46.6 ± 8.5, respectively). The duration of renal disease was calculated from the first diagnosis of renal impairment and was found to be 47.83 ± 12.24 months. Data of studied groups is summarized in
Table 1 Data of studied groups
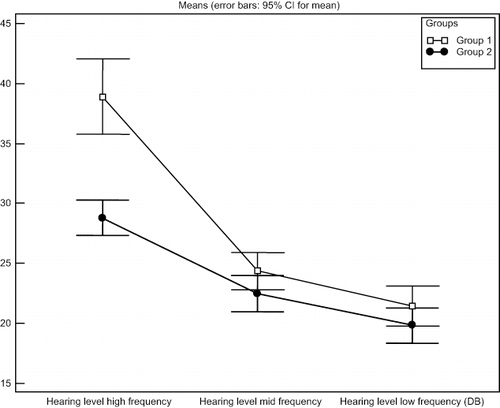
The hearing level at different frequencies is presented in , and shows hearing level at different frequencies in both groups (mean and range). The etiology of renal disease in group one is presented in .
Table 2 Hearing level at different frequencies for studied group
Table 3 Etiology of renal disease in group 1
No correlation was found between the stage of renal insufficiency and hearing loss, Pearson chi-square = 0.71 for low frequency, and 0.22 for mid frequency hearing loss. No correlation was found between ADMA level and stage of renal disease, Pearson chi-square = 0.37.
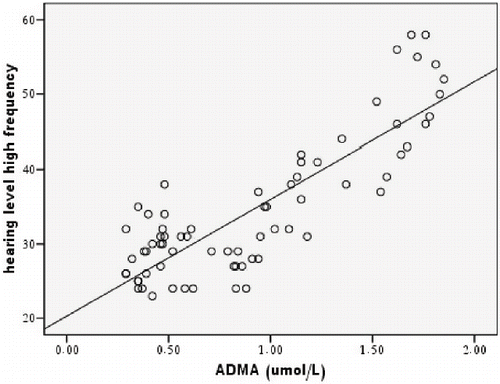
Multiple regression analysis for hearing level at high frequency in group one shows that significant independent predictors of hearing level are ADMA (t = 3.37, p = .002), high sensitivity CRP (t = 2.4, p = 0.02), duration of renal disease (t = −2.6, p = 0.01), presence of diabetes mellitus (t = 4.5, p < 0.001), and serum creatinine (t = 2.8, p = 0.008). However, no significance is found with age (p = 0.59), gender (p = 0.27), smoking (p = 0.4), hypertensive kidney disease (p = 0.63), hematocrit (p = 0.23), HDL (p = 0.35), total cholesterol (p = 0.09), and triglyceride (p = 0.46). shows a scatterplot of hearing level at high frequency in patients with renal impairment and ADMA level.
Figure 2. Scatterplot for hearing level at high frequency and ADMA level in patients with renal impairment.
Upon comparing hearing level at high frequency in diabetic and non-diabetic patients with renal impairment, a significance difference is found (t = 7.5, p < 0.001). Also, smokers appear to have significantly higher hearing level at the same frequency than non-smokers (t = 5.0, p > 0.001), yet multiple regression analysis failed to show smoking as an independent predicting factor for hearing level.
DISCUSSION
The problem of hearing loss in patients with chronic renal failure was previously reported in literature with different frequencies. The majority of the studies included patients on hemodialysis and few studies on peritoneal dialysis patients. Fewer studies were carried out on adult patients with renal impairment under conservative treatment. Many explanations had been put forward for hearing loss in patients with CRF, but the exact etiology and pathogenesis is not yet known.
We studied hearing level at different frequencies using pure tone audiometry. Warady et al.Citation[15] stated that PTA is a better indicator of hearing function than click-evoked ABR in patients with CRF.
Our results show a high frequency hearing loss in patients with renal impairment compared to normal control subjects. Similar results were obtained by Zeigelboim et al., who found a severe high-frequency hearing loss in the group with CRF undergoing conservative treatment.Citation[16] Also, Gafter et al. described sensorineural hearing loss in CKD patients on conservative treatment.Citation[17] Mancini et al. suggested that a neural conduction along the auditory pathway is delayed irrespective of hemodialysis onset, basically due to the CRF, thus attributing the hearing loss to uremia.Citation[18]
We found that hearing level at mid and low frequencies are not statistically different from those of the control group, although the mean value is slightly higher in patients with CRF. This is in contrast to Bains et al., who found that chronic kidney disease patients have a highly significant bilateral sensorineural hearing loss at all frequencies of 0.25 to 8.0 kHz, though more marked in higher frequencies.Citation[19]
There was a debate about the relationship between renal insufficiency and hearing loss, whether the condition is related to age or accelerated presbycusis. Bains et al. found that hearing loss in patients with CRF is partially reversible after successful renal transplantation. Their results indirectly pointed toward the uremic milieu being the cause, in view of some improvement after renal transplantation.Citation[19]
Multivariate analysis shows no significant correlation between high frequency hearing level in patients with CRF with gender, smoking, hypertension, hematocrit, HDL, total cholesterol, and triglyceride level. Kusakari et al. reported that inner ear dysfunction (including hearing loss and vestibular dysfunction or a combination) was not correlated with hematocrit, BUN, or serum creatinine.Citation[20] Also, Jorgenson found that hearing loss was not related to changes in creatinine, K+, Na+, Ca++, glucose, BUN, blood pressure, weight, or hyperlipidemia.Citation[21]
Multivariate analysis of our data shows that hearing loss at a high frequency in patients with renal impairment is independently determined by duration of renal disease. This matches with Bains et al.Citation[19] and Zeigelboim et al., who stated that hearing loss among patients with CRF seemed to deteriorate further a year after the first evaluation.Citation[16] This is in contrast to Henrich et al., who found that hearing loss is common in renal failure, but it does not worsen with duration of treatment.Citation[22]
Our results show that the degree of hearing loss is related to serum creatinine. This is a similar finding to that by Risvi et al., who reported progressive hearing loss parallel to progression of CRF.Citation[23] However, Kusakari et al. reported that inner ear dysfunction was not correlated with BUN or serum creatinine levels.Citation[20]
Our results show that the presence of diabetes is one of the contributing factors for hearing loss in patients with CRF. Studies about the relationship between diabetes mellitus and auditory impairment have shown variable results.Citation[24] Studies of auditory function taking into account blood glucose control and other complications of the disease are scarce. Luz Ver et al. stated that Type 2 diabetes mellitus can have subclinical hearing loss and impaired auditory brainstem response, independent of peripheral neuropathy, retinopathy or nephropathy. This is related to age of the patients, as diabetes may accelerate age-related hearing loss.Citation[25] Thus, the presence of diabetes may potentiate the detrimental effects of chronic renal failure on hearing.
We found that hearing loss in patients with CRF is related to high sensitivity CRP, which suggests an inflammatory role in the pathogenesis of hearing loss. Several ABR (auditory brainstem response) studies of CRF indicated dysfunction of the auditory nerve and pathways.Citation[4] Thus, the auditory nerve may be involved in uremic neuropathy like peripheral nerves, which are affected by chronic inflammation and uremic toxins.Citation[26] Also, inflammatory endothelial activation and endothelial cell dysfunction are some of the underlying causes for the small-vessel disease affecting the cochlea and auditory pathway.Citation[27]
Our results show that the degree of high-frequency hearing loss is independently affected by ADMA level. To our knowledge, there are no previous reports correlating hearing disorder in patients with renal failure with ADMA. Our finding do not prove whether elevated ADMA levels is the cause of hearing loss. However, a close relationship of ADMA to hearing loss suggests the role of elevated level of ADMA in the pathogenesis of hearing loss in patients with CRF.
The mechanism by which ADMA can contribute to hearing disorder in our patients is not clear. There are many explanations for this relation. It is known that hemorheological variations due to alterations of blood cells and plasma components can lead to hyperviscosity, which may slow microcirculation to hearing organs. Nitric oxide released by the endothelium in response to shear stress plays a crucial role in flow-mediated vasodilatation.Citation[28]
Changes in viscosity, blood clotting, and fibrinolysis may contribute, at least in part, to the pathophysiological mechanism of sensorineural hearing loss. The more recent studies performed in patients affected by idiopathic sensorineural hearing loss have shown an effect of hemodilution alone as a possible therapeutic option.Citation[29]
ADMA caused by the impairment of NO availability, which affects red blood cell deformability and blood viscosity, can possibly contribute to microcirculatory alterations to hearing organs, such as those found in idiopathic sensorineural hearing loss.Citation[30]
Charachon et al.Citation[31] attributed the high incidence of hearing loss in CRF to the premature aging caused by the disease. Serum ADMA is known to affect vascular disease in CRF and correlates with the abnormal thickening of the carotid artery in CRF patients. This vascular disease can cause premature aging in patients with CRF.Citation[32] Also, vascular change with ischemia to cochlea and post-cochlear structures had been listed as one of the pathologic changes causing sensorineural hearing loss.Citation[33]
ADMA causes an increase in systemic and renal vascular resistance, a reduction in vascular compliance, and an attenuation of cerebral blood flow.Citation[34] Whether this chronic reduction in cerebral flow affects the labyrinthine system, causing hearing disorders, needs further study.
Antonelli et al.Citation[35] found that patients with CRF had a significantly subclinical dysfunction of the VIII nerve caused by axonal uremic neuropathy. ADMA was found to contribute to the development of neuropathy, as lowering its level leads to an improvement in different nerve functions.Citation[36]
Hearing loss is linked to renal failure irrespective of the treatment method.Citation[37] Thus, the causative factors for hearing loss are expected to be present and not modified by modality of replacement therapy. ADMA is one of the uremic toxins that increases early in the course of CRF, even before a reduction in GFR, ensues and continues to rise in ESRD with little effect of HD and peritoneal dialysis.Citation[38] Thus, ADMA may be one of the uremic toxins contributing to hearing disorder in CRF patients.
CONCLUSION AND CLINICAL IMPLICATIONS
The exact cause of hearing loss in patients of renal failure is not yet known. Our results point to the possibility of the involvement of ADMA in the etiology of this common complication. We found that high-frequency hearing loss is common in patients with renal impairment before start of replacement therapy. We also found that hearing loss is related to the duration of renal disease, serum creatinine, presence of DM, HsCRP level, and ADMA levels. There is little to be done for those patients for whom the modality of renal replacement therapy did not show any favorable effect on hearing loss. A therapeutic approach that can reduce ADMA level can be beneficial in controlling this complication. A well-controlled approach and multicenter interventional studies are required to explore the role of ADMA in hearing loss in patients with CRF and the possible therapeutic effects of different drugs that are shown to reduce ADMA level.
There are different therapeutic approaches that are known to reduce ADMA level, such as improved glycemic control with metformin, rosuvastatin, estrogen therapy in postmenopausal woman, thyroxine replacement in patients with subclinical hypothyroidism,Citation[38] and treatment aimed at reducing oxidative stress.Citation[39] A new, specific drug that increases DDAH expression and lowers ADMA, the farnesoid X receptor agonist GW 4064, is still in an early stage of development.Citation[40] However the beneficial effect of these drugs on hearing loss in patients with CRF needs further study.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Bergstrom L, Jenkins P, Sando I, G E. Hearing loss in renal disease. Clinical and Pathological Studies. Ann Oto Rhino Lary 1973; 82: 555–574
- Brookes GB, Vitamin D. deficiency and deafness. Am J Otol. 1985; 6: 102–107
- Adler D, Fiehn W, Ritz E. Inhibition of Na+, K+-stimulated ATPase in the cochlea of the guinea pig. A potential cause of disturbed inner ear function in terminal renal failure. Acta Otolaryngol. 1980; 90: 55–60
- Orendorz-Fraczkowska K, Makulska I, Pospiech L, Zwolinska D. The influence of haemodialysis on hearing organ of children with chronic renal failure. Otolaryngol Pol. 2002; 56: 597–602
- Kakimoto Y, Akazawa S. Isolation and identification of N-G, N-G- and N-G,N’-G-dimethyl- arginine,N-epsilon-mono-, di-, and trimethyllysine, and glucosylgalactosyl- and galactosyl-delta-hydroxylysine from human urine. J Biol Chem. 1970; 245: 5751–5758
- Murray-Rust J, Leiper J, McAlister M, Phelan J, Tilley S, Santa Maria J. Structural insights into the hydrolysis of cellular nitric oxide synthase inhibitors by dimethylarginine dimethylaminohydrolase. Nat Struct Biol 2001; 8: 679–683
- Thum T, Tsikas D, Stein S, et al. Suppression of endothelial progenitor cells in human coronary artery disease by the endogenous nitric oxide synthase inhibitor asymmetric dimethylarginine. J Am Coll Cardiol. 2005; 46: 1693–1701
- Fliser D. Asymmetric dimethylarginine (ADMA): The silent transition from an ‘uraemic toxin’ to a global cardiovascular risk molecule. European Journal of Clinical Investigation. 2005; 35: 71–79
- Vallance P, Leone A, Calver A. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992; 339: 572–575
- Ghiadoni L, Cupisti A, Huang Y. Endothelial dysfunction and oxidative stress in chronic renal failure. J Nephrol. 2004; 17: 512–519
- Kielstein JT, Bode-Boeger SM, Hesse G, Martens-Lobenhoffer J, Fliser D, Hoeper MM. ADMA in idiopathic pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol. 2005; 25: 570–574
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972; 18: 499–502
- Macy EM, Hayes TE, Tracy RP. Variability in the measurement of C-reactive protein in healthy subjects: Implications for reference intervals and epidemiological applications. Clin Chem. 1997; 43: 52–58
- Chen BM, Xia LW, Zhao RQ. Determination of N(G),N(G)-dimethylarginine dimethylarginine in human plasma by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 1997; 692: 467–471
- Warady BA, Reed L, Murphy G, et al. Aminoglycoside ototoxicity in pediatric patients receiving long-term peritoneal dialysis. Pediatric Nephrol. 1993; 7: 178–181
- Zeigelboim B, Mangaberia-Albernaz P, Fukuda Y. High frequency audiometry and chronic renal failure. Acta Otolaryngol. 2001; 121: 245–248
- Gafter U, Shvili Y, Levi J, et al. Brainstem auditory evoked responses in chronic renal failure and the effect of hemodialysis. Nephron 1989; 53
- Mancini M, Dello Strologo L, Bianchi P, Tieri L, Rizzoni G. Sensorineural hearing loss in patients reaching chronic renal failure in childhood. Pediatr Nephrol. 1996; 10: 38–40
- Bains KS, Chopra H, Sandhu JS, Aulakh BS. Cochlear function in chronic kidney disease and renal transplantation: A longitudinal study. Transplant Proc. 2007; 39: 1465–1468
- Kusakari J, Kobayashi T, Rokugo M, et al. The inner ear dysfunction in hemodialysis patients. Nephrology Dialysis Transplantation. 1981; 135: 359–369
- Jorgenson MG. Changes in aging in the inner ear and the inner ear in diabetes mellitus. Histologic studies. Acta Otolaryngol. 1963; 188: 125–128
- Henrich W, Thompson P, Bergstrom L, Lum GM. Effect of dialysis on hearing acuity. Nephron. 1977; 18: 348–351
- Risvi SS, Holmes RA. Hearing loss from hemodialysis. Arch Otolaryngol. 1980; 106: 751–756
- deEspana R, Biurrun O, Lorente J, Traserra J. Hearing and diabetes. ORL J Otorhinolaryngol Relat Spec. 1995; 57: 325–327
- Diaz de Leon-Morales Luz V, Jauregui-Renaud K, Garay-Sevilla ME, Hernandez-Prado J, Malacara-Hemandez JM. Auditory Impairment in patients with type 2 diabetes mellitus. Archives of Medical Research. 2005; 36: 507–510
- Di Paolo B, Di Marco T, Cappelli P, et al. Electrophysiological aspects of nervous conduction in uremia. Clin Nephrol. 1988; 29: 253–260
- Hassan A, Hunt BJ, O'Sullivan M, et al. Markers of endothelial dysfunction in lacunar infarction and ischaemic leukoaraiosis. Brain. 2003; 126: 424–432
- Cooke JP, Rossitch E, Jr, Andon NA, Loscalzo J, Dzau VJ. Flow activates an endothelial potassium channel to release an endogenous nitrovasodilator. J Clin Invest. 1991; 88: 1663–1671
- Ziegler EA, Hohlweg-Majert B, Maurer J, Mann WJ. Epidemiological data of patients with sudden hearing loss—a retrospective study over a period of three years. Laryngorhinootologie. 2003; 82: 4–8
- Mannini L, Cecchi E, Fatini C, et al. Clinical haemorheology and microcirculation. Ann Ist Super Sanità. 2007; 43: 144–155
- Charachon R, Moreno-Ribes V, Cordonnier D. Deafness due to renal failure. Clinicopathological study. Ann Otolaryngol Chir Cervicofac. 1978; 95: 179–203
- Zoccali C, Benedetto FA, Maas R, et al. Asymmetric dimethylarginine, C-reactive protein, and carotid intima-media thickness in end-stage renal disease. J Am Soc Nephrol. 2002; 13: 490–496
- Markus C, Frantz A, Bertram F, Pontz B, Hyperbaric Arnold W. oxygen treatment restores sudden hearing loss in a patient with Fabry disease. ORL J Otorhinolaryngol Relat Spec. 2008; 70: 210–213
- Kielstein JT, Donnerstag F, Gasper S, et al. ADMA increases arterial stiffness and decreases cerebral blood flow in humans. Stroke. 2006; 37: 2024–2029
- Antonelli A, Bonfiolii F, Garrubbaa V, et al. Audiological findings in elderly patients with chronic renal failure. Acta Otolaryngologica. 1991; 476: 54–68
- Chan NN, Vallance P. Hyperhomocysteinaemia and neuropathy in Type 2 diabetes. Diabetic Medicine. 2001; 18: 1008–1009
- Thodi C, Thodis E, Danielides V, Pasadakis P, Vargemezis V. Hearing in renal failure. Nephrol Dial Transplant. 2006; 21: 3023–3030
- Kielstein JT, Fliser D. The past, presence and future of ADMA in nephrology. Néphrologie & Thérapeutique. 2007; 3: 47–54
- Saran R, Novak JE, Desai A, et al. Impact of vitamin E on plasma asymmetric dimethylarginine (ADMA) in chronic kidney disease (CKD): A pilot study. Nephrol Dial Transplant. 2003; 18: 2415–2420
- Hu T, Chouinard M, Cox AL, et al. FXR agonist reduces serum asymmetric dimethylarginine levels through hepatic dimethylarginine dimethylaminohydrolase-1 gene regulation. J Biol Chem 2006; 281: 39831–39838