Abstract
Introduction. Several natural products have been reported to have beneficial effects on ischemia/reperfusion (I/R) injury, particularly from a preventative perspective. Therefore, this study was designed to investigate the efficiency of proanthocyanidin (PA), a natural product derived from grape seed, on renal dysfunction and injury induced by I/R of rat kidney. Materials and Methods. Twenty-four male Sprague-Dawley rats were divided into three groups: sham-operated, I/R, I/R+PA. Rats were given PA (100 mg/kg/day peroral) 7 days prior to I/R. All rats except sham-operated underwent 60 min of bilateral renal ischemia followed by 6 h of reperfusion. After reperfusion, kidneys and blood were obtained for evaluation. Superoxide dismutase, glutathione peroxidase, malondialdehyde, protein carbonyl content, and nitrite/nitrate level (NOx) were determined in the renal tissue. Serum creatinine (SCr), blood urea nitrogen (BUN), and aspartate aminotransferase (AST) were determined in the blood. Additionally, renal sections were used for histological grade of renal injury. Results. PA significantly reduced the I/R-induced increases in SCr, BUN, and AST. In addition, PA markedly reduced elevated oxidative stress product, restored decreased antioxidant enzymes, and attenuated histological alterations. Moreover, PA attenuated the tissue NOx, levels indicating reduced NO production. Conclusions. The pretreatment of rats with PA reduced the renal dysfunction and morphological changes, ameliorated cellular injury, and restored renal antioxidant enzymes caused by renal I/R.
INTRODUCTION
The cessation of kidney function leads to renal failure. Acute renal failure (ARF), which occurs in transplantation, shock, sepsis, and renal artery stenosis, involves the failure of the kidneys over a period of hours or days.Citation[1],Citation[2] The causes of ARF are often multifactorial, but can be classified into three categories, depending on cause: prerenal ARF, intrinsic ARF, and postrenal ARF. In the clinical setting, ischemic ARF is often caused by hypotension followed by resuscitation, the etiology of which is reflected in animal models of renal ischemia/reperfusion (I/R).Citation[3]
Ischemia (the cessation of blood flow), followed by reperfusion (the re-establishment of blood flow), causes characteristic injury to kidneys. Reperfusion of tissue or the kidney after ischemic period causes the release of proinflammatory substances and the formation of both nitrogen-derived (reactive nitrogen species [RNS]) and oxygen-derived (reactive oxygen species [ROS]) free radicals, such as superoxide (O2¯), peroxide (H2O2), and hydroxyl radicals (OH¯).Citation[2–4] These free radicals are normally removed by antioxidant enzymes, mainly superoxide dismutase (SOD) and gluthationeperoxidase (GSH-Px). I/R injury can be evaluated by the detection of various products resulting from injury, using laboratorial and histomorphological methods. Malondialdehyde (MDA), frequently used to show the involvement of free radicals in cell damage, is one of the final products of lipid peroxidation. Besides lipid peroxidation, the measurement of protein damage by protein carbonyl content (PCC) and antioxidant enzyme activities, can be performed to quantify ROS/RNS in laboratory.Citation[5–8]
Several investigations regarding the implication of ROS on renal I/R have demonstrated the beneficial effects of pharmacological interventions, such as the prevention of ROS generation,Citation[9] inhibition of enzymes responsible for ROS generation,Citation[10] administration of antioxidant enzymes,Citation[11] and scavenging of ROS molecules.Citation[12] Although some pharmacological interventions have provided promising results against renal I/R injury, the potential benefits of systemic clinical administration of these agents have been limited due to several confounding factors. On the other hand, there is growing interest in natural products as agents to manage health, particularly from a preventative perspective. Several plant-derived agents have been reported to have beneficial effects on renal I/R injury.
Proanthocyanidin (PA) is powerful naturally occurring polyphenolic antioxidant widely available in fruits, vegetables, grape seeds, nuts, and flowers.Citation[13],Citation[14] PA has been demonstrated to exert a novel spectrum of biological, pharmacological, and therapeutic properties against ROS and oxidative stress.Citation[15],Citation[16] Thereby, the potential influence of PA on renal antioxidant enzyme activity, tissue damage, and histopathological changes were investigated.
MATERIALS AND METHODS
The project was approved by the Experimental Ethics Committee of Gulhane Military Medical Academy, Ankara, Turkey, and the National Institute of Health's Guide for the Care and Use of Laboratory Animals was followed.
Surgery and Experimental Protocol
Twenty-four male Sprague-Dawley rats, weighing 250–300 g, were provided by the Gulhane Military Medical Academy, Experimental Research Council, and housed in standard cages at a constant temperature (24oC), humidity (70%), and light-dark cycle in a controlled environment. Rats were under standard rat chow and water ad libitum.
Rats were randomly divided into three groups: sham-operated (n = 8), renal I/R (n = 8), and renal I/R+PA (n = 8). The IR+PA group was given proanthocyanidin (100 mg/kg per day for 7 days) orally prior to experiment. Proanthocyanidins derived from grape seeds were obtained from General Nutrition Centers, Inc. (GNC; Pittsburgh, Pennsylvania, USA). At the end of the treatment period, animals were anesthetized with an intraperitoneal injection of ketamine hydrochloride (50 mg/kg) and xylazine (10 mg/kg). The rats were placed on a heating pad kept at 39°C to maintain constant body temperature. A midline incision was made, the renal pedicle observed, and arteries bilaterally occluded with an atraumatic microvascular clamp (Vascustatts II, midi 1001–532, Scatlan, Minnesota, USA) for 60 min. The time of ischemia was chosen to maximize reproducibility of renal functional impairment while minimizing mortality in these animals. After 60 min of renal ischemia, the clamps were removed, and the kidneys were inspected for restoration of blood flow. The abdomen was closed in two layers. Sham-operated animals underwent the same surgical procedure without clamp application. Following 6 h of reperfusion period, animals were killed by cervical dislocation. At the time of death, blood was collected by heart puncture for measurement of biochemical analysis. Both kidneys were harvested for histopathological evaluation and biochemical examination.
Biochemical Analysis
Biochemical Parameters
Serum samples were used for the measurement of blood urea nitrogen (BUN) and serum creatinine (SCr) levels, which were used as indicators of impaired glomerular function, and aspartate aminotransferase (AST), which was used as an indicator of renal I/R injury.Citation[17] BUN, SCr, and AST were determined with an Abbott-Aeroset autoanalyzer (Chicago, Illinois, USA) using original kits.
Tissue Preparation
The frozen tissues were homogenized in phosphate buffer (pH 7.4) by means of a homogenizator (Heidolph Diax 900; Heidolph Elektro GmbH, Kelhaim, Germany) kept on ice. The supernatant was stored at -70oC.
Proteins Assay
Initially, the protein content of tissue homogenates was measured by the method of Lowry et al.Citation[18] with bovine serum albumin as the standard.
Determination of Tissue Protein Carbonyl Content (PCC)
The tissue PCC was determined spectrophotometrically by the method based on the reaction of the carbonyl group with 2, 4-dinitrophenylhydrazine to form 2, 4-dinitrophenylhydrazone.Citation[19] 2, 4-Dinitrophenylhydrazine was the reagent originally used for proteins subjected to metal-catalyzed oxidation. Absorbances were measured with a spectrophotometer (Helios Epsilon, UNICAM, Cambridge, UK). The results were given as millimoles carbonyl per gram protein.
Determination of Tissue Lipid Peroxidation Level (MDA)
Lipid peroxidation level was measured with the thiobarbituric acid (TBA) reaction using the method described by Ohkawa.Citation[20] This method was used to obtain a spectrophotometric measurement of the color produced during the reaction to thiobarbituric acid (TBA) with MDA at 535 nm. MDA levels were expressed as mmol/g protein.
Determination of Superoxide Dismutase Activity (SOD)
SOD activity was assayed using the nitroblue tetrazolium (NBT) method of Sun et al.Citation[21] NBT was reduced to blue formazan by O2¯, which has a strong absorbance at 560 nm. One unit (U) of SOD is defined as the amount of protein that inhibits the rate of NBT reduction by 50%. The calculated SOD activity was expressed as U/gprotein.
Determination of Glutathione Peroxidase (GSH-Px)
The GSH-Px activity was measured using the method described by Paglia and ValentineCitation[22] in which GSH-Px activity was coupled with the oxidation of NADPH by glutathione reductase. The oxidation of NADPH was spectrophotometrically followed up at 340 nm at 37°C. The absorbance at 340 nm was recorded for 5 min. The activity was the slope of the lines as mmol of NADPH oxidized per minute. GSH-Px activity was presented as U/g protein.
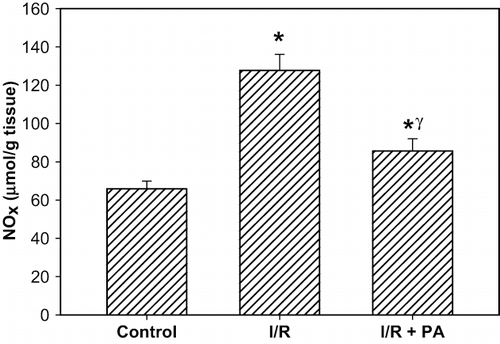
Determination of Nitrate and Nitrite (NOx)
Nitrate and nitrite (NOx) activity was measured using the method described by Miranda et al.Citation[23] Tissue homogenates centrifuged at 2000 × g for 5 min. The supernatant (0.5 ml) was added to 0.25 ml 0.3 M NaOH. After incubation for 5 min at room temperature, 0.25 ml of 5% (w/v) ZnSO4 was added for deproteinization. This mixture was then centrifuged at 3000 × g for 20 min (+4°C), and the supernatants were used for the assays.
A nitrate standard solution (100 μl) was serially diluted (generally from 200–1.6 mM) in duplicate in a 96-well plate. After loading the plate with samples (100 μl), the addition of VCl3 (100 μl) to each well was rapidly followed by addition of the Griess reagents, sulfanilamide (SULF) (50 μl), and N-(1-Naphthyl)ethylenediamine (NEDD) (50 μl). The Griess solutions may also be premixed immediately prior to application to the plate. Sample blank values were obtained by substituting diluting medium for Griess reagent.
Nitrite was measured in a similar manner except that samples and nitrite standards were only exposed to Griess reagents. In either case, the absorbance at 540 nm was measured using a plate reader following incubation (30 min).
Tissue NOx levels were expressed as μmol/g tissue.
Histopathologic Evaluation
Tissue specimens were fixed in 10% formalin and embedded in paraffin and cut into 5 μm sections. Slides were prepared with hematoxylin and eosin (H&S) stain and examined under light microscopy. Each slide was evaluated in a blind manner by two separate investigators for tubular cell swelling, brush border loss, nuclear condensation, and nuclear loss. One hundred intersections were examined for each kidney. Injury was graded on a four-tiered scale as follows:
grade 0 represent no diagnostic change;
grade 1 demonstrates tubular cell swelling, brush border loss, and nuclear condensation, with up to one-third of the tubular profile showing nuclear loss;
grade 2 is the same as grade 1, but with greater than one-third and less than two-thirds of the tubular profile showing nuclear loss;
grade 3 displays greater than two-thirds of the tubular profile showing nuclear loss.
The total score for each kidney was calculated by the addition of all 100 scores, with a maximum score of 300.Citation[17]
Statistical Analysis
All data are expressed as mean ± standard error of the mean (SEM). All statistical analyses were carried out using SPSS statistical software (SPSS for Windows, Version 15.0, Chicago, Illinois, USA). Differences in measured parameters among the three groups were analyzed by Kruskal-Wallis test. Dual comparisons between groups that present significant values were evaluated with Mann-Whitney U test. Statistical significance was accepted a value of p < 0.05.
RESULTS
Renal Function Markers
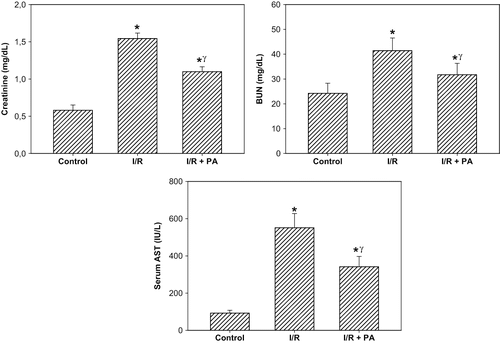
There was a significant increase in the SCr and BUN levels in the I/R group compared to the sham-operated group, suggesting a significant degree of glomerular dysfunction (p < 0.01; see ). Regular intake of PA prior to I/R produced a slight, but statistically significant, reduction in the SCr and BUN levels in the I/R+PA group compared to the I/R group (p < 0.05). Whereas renal I/R produced a significant increase in the serum AST level, used as a marker of I/R injury, in the I/R group, serum concentration of AST was significantly reduced in the rats receiving PA (p < 0.01, I/R+PA vs. I/R group; see ).
Figure 1. Effect of renal ischemia reperfusion (I/R) and proanthocyanidin (PA) on serum creatinine, blood urea nitrogen (BUN), and serum aspartate aminotransferase (AST) levels. All values expressed as mean SEM. *statistically significant from control (p < 0.05); γstatistically significant from I/R group (p < 0.05).
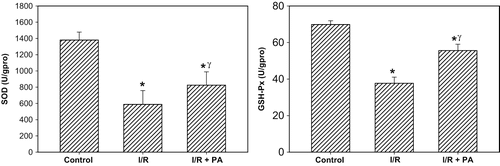
Antioxidant Enzyme Activities
Antioxidant enzyme activities are displayed in . SOD and GSH-Px were significantly the lowest in the I/R group (p < 0.001, I/R group vs. sham-operated and I/R+PA group). On the other hand, antioxidant enzyme activities were significantly increased in rats receiving PA (p < 0.01, I/R+PA group vs. I/R group).
Figure 2. Effect of renal ischemia reperfusion (I/R) and proanthocyanidin (PA) on tissue superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) enzyme activities. All values expressed as mean SEM. *statistically significant from control (p < 0.05); γstatistically significant from I/R group (p < 0.05).
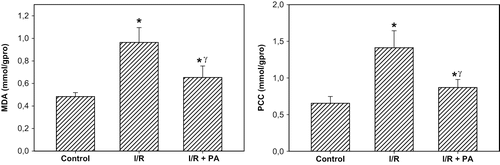
Oxidative and Nitrosative Stress Markers
The rats subjected to renal I/R revealed a strike increase in tissue MDA and PCC levels (p < 0.001, I/R group vs. I/R+PA and sham-operated group), suggesting increased lipid peroxidation and protein oxidation. Regular intake of PA exhibited a decrease the levels of MDA and PCC in the rats (p < 0.01, I/R+PA group vs. I/R group; see ).
Figure 3. Effect of renal ischemia reperfusion (I/R) and proanthocyanidin (PA) on tissue malonedialdehyde (MDA) and protein carbonyl content (PCC). All values expressed as mean SEM. *statistically significant from control (p < 0.05); γstatistically significant from I/R group (p < 0.05).
NOx level (nitrite/nitrite concentration) in the renal tissue, as indicator of NO synthesis, was significantly increased in the rats subjected to renal I/R (p < 0.001, I/R group vs. I/R+PA and sham-operated group). Increased tissue NOx levels were significantly decreased in the I/R+PA group (p < 0.01, IR/PA vs. I/R group; see ).
Histopathologic Evaluation
Histological grading of renal injury is displayed in . The total histological injury score was significantly increased in the I/R group, indicating significant tubular injury (p < 0.01, I/R group vs. sham-operated and I/R+PA group). Total histological injury score was significantly decreased in the rats received PA (p < 0.05, I/R+PA vs. I/R group). Representative histological samples from all groups are displayed in .
Figure 5. Effect of renal ischemia reperfusion (I/R) and proanthocyanidin (PA) on the total renal histological injury score. All values expressed as mean SEM. *statistically significant from control (p < 0.05); γstatistically significant from I/R group (p < 0.05).
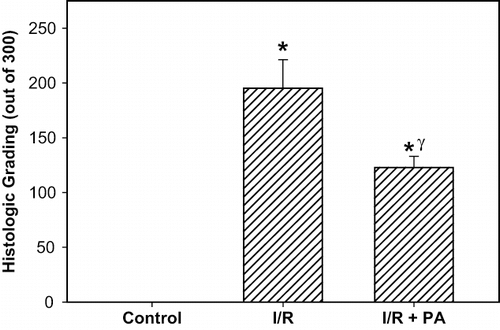
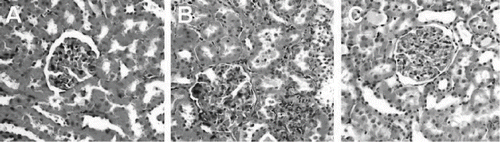
Figure 6. H&E stained of rat kidneys: (A) kidney section of sham-operated rat showing glomeruli and tubules appear normal; (B) kidney section of rat subjected to ischemia reperfusion (I/R) showing tubular cell swelling, nuclear condensation, and tubular dilatation; (C) kidney section of PA-treated rat showing minimal tubular cell swelling, brush border loss, and nuclear condensation. Hematoxylin and eosin; original magnification ×40) .
DISCUSSION
We have investigated the potential renoprotective effects of PA in a rat model of renal I/R injury. Our findings show that PA reduces renal glomerular and tubular dysfunction in rats' kidneys subjected to I/R injury. Moreover, PA has an ameliorating effect on both oxidative stress and nitrosative stress of the kidneys. In addition, histopathological evaluation of the kidneys also confirms the protective effect of PA. The implications of these findings are important.
Renal I/R causes both glomerular and tubular dysfunction.Citation[2],Citation[3] The present study clearly revealed that 60 min ischemia following 6 h reperfusion increased SCr and BUN levels, indicating significant impairment of glomerular function. We also found that renal I/R produced a significant increase in the serum AST level, used as a marker of renal I/R injury. Biochemical evidence of tubular injury was also supported by total histological scoring of kidneys. Based on these findings, our experimental model can be considered to be reliable to evaluate the efficacy of our drug on renal I/R injury. In addition, the present study clearly revealed that intake of PA has significant beneficial effects on the reduction of renal dysfunction and injury mediated by I/R of the kidneys.
We measured antioxidant enzyme activities (SOD and GSH-Px) to evaluate the tissue antioxidant system, and it was demonstrated that I/R significantly decreased SOD and GSH-Px enzyme activities of the kidneys. On the other hand, the administration of PA significantly increased the antioxidant enzymes activities, suggesting that this agent scavenged free radicals in the kidneys. Several experimental studies suggest that PA may prevent or reduce cell and tissue damage of the liver, kidney, and brain, and myocardium induced oxidative stress.Citation[13],Citation[24],Citation[25] In vitro investigation of antioxidant potential of PA (grape seed extract) shows that it is a strong antioxidant, up to five times more effective than vitamin C or E.Citation[16] Moreover, it is known that flavonoids (e.g., PA) have a potential function in the transfer of electrons between their isoalloxazine group and cell reactants in oxidation-reduction reactions to render them less reactiveCitation[13],Citation[14] and can chelate metals like iron involved in free radicals formation.Citation[14],Citation[26] Flavonoids inhibit many enzymes, including cyclooxygenase, lipoxygenase,Citation[27] and phospholipase A2,Citation[13],Citation[28] which play significant roles in the generation of ROS. It has also been shown that PA could prevent the reduction of catalase and SOD activities in pesticide exposure.Citation[29] Thus, we speculate that increased antioxidant enzyme activities in rats receiving PA might be attributed to the scavenging of ROS/RNS from the environment by an interaction of PA with ROS.
Additionally, we monitored the tissue injury indices (MDA and PCC) to evaluate the oxidative stress status in the kidneys. We found that IR caused a considerable increase in the kidneys levels of MDA and PCC. This is consistent with previous reports that the peroxidation of cellular membrane lipids and oxidation of cellular proteins, determined by MDA and PCC, respectively, increases by ROS/RNS following I/R injury.Citation[3],Citation[8],Citation[30–33] In addition, we found that the oral supplementation of rats with PA prior to I/R significantly reduced the extent of lipid peroxidation and protein oxidation in the kidneys, indicating decreased cellular injury. Protection of cells might be the consequence of molecular structure of PA, which allows them to accumulate at lipid interfacesCitation[28] and/or to penetrate into membranes.Citation[26] Additional support for the effect of PA in decreasing lipid peroxidation is reported by Roig et al.,Citation[25] who showed that PA is a powerful protective agent against H2O2-induced hepatocellular lipid peroxidation. The cytoprotective efficacy of PA was also tested on acetaminophen-induced hepato and nephrotoxicity, amiodarone-induced pulmonary toxicity, and doxorubicin-induced cardiotoxicity in mice.Citation[13],Citation[24],Citation[34] Therefore, it can be concluded that PA might protect cellular membranes of kidneys from oxidative damage by acting as a powerful antioxidant and by restoring membrane components and/or modifying membrane components.
In this work, PA effectively inhibited an I/R-induced rise in tissue NOx concentration. NO produced by iNOS reacts rapidly with O2¯ to produce the highly reactive peroxynitrite anion (ONOO¯). NO and ONOO¯ are eventually converted to nitrite (NO2) and/or nitrate (NO3), and nitrite plus nitrate is known as NOx. Therefore, tissue NOx levels (tissue nitrite/nitrate) can be used as an indirect but reliable indicator for NO and ONOO¯ formation in vivo.Citation[35],Citation[36] NO produced by iNOS during renal I/R contributes to renal injury. The role of NO in the development of renal I/R injury has been confirmed in several investigations where the administration of iNOS inhibitors protect the kidney against I/R injury.Citation[17],Citation[37–40] In two studies, PA oligomers were tested for their ability to protect against ONOO¯-dependent oxidation, and it was suggested that PA do not directly react with ONOO¯ but most likely react with oxidizing/nitrating intermediates.Citation[41],Citation[42] On the basis of a correlation between decreased tissue NOx levels and histological injury scores in our work, it is conceivable that renal I/R injury and ARF originating from the excessive production of NO and PA may have an inhibitory effect on iNOS and/or ONOO¯ production.
In conclusion, based on our observations, PA prevented I/R injury in the kidneys by decreasing oxidative and nitrosative stress. Our study provides experimental data indicating that PA is highly bioavailable and may serve as a potential therapeutic agent in protecting kidney and multiple target organs from I/R injury. Human clinical trials are needed to reveal further benefits of this strong natural antioxidant in the renal I/R injury and ARF.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Rondon-Berrios H, Palevsky PM. Treatment of acute kidney injury: An update on the management of renal replacement therapy. Curr Opin Nephrol Hypertens 2007; 16: 64–70
- Sheridan AM, Bonventre JV. Pathophysiology of ischemic acute renal failure. Contrib Nephrol 2001; 132: 7–21
- Chatterjee PK. Novel pharmacological approaches to the treatment of renal ischemia-reperfusion injury: A comprehensive review. Naunyn Schmiedebergs Arch Pharmacol 2007; 376: 1–43
- Nath KA, Norby SM. Reactive oxygen species and acute renal failure. Am J Med 2000; 109: 665–678
- Cerqueira NF, Hussni CA, Yoshida WB. Pathophysiology of mesenteric ischemia/reperfusion: A review. Acta Cir Bras 2005; 20: 336–343
- Erdogan H, Fadillioglu E, Yagmurca M, Ucar M, Irmak MK. Protein oxidation and lipid peroxidation after renal ischemia-reperfusion injury: Protective effects of erdosteine and N-acetylcysteine. Urol Res 2006; 34: 41–46
- Harris AG, Leiderer R, Peer F, Messmer K. Skeletal muscle microvascular and tissue injury after varying durations of ischemia. Am J Physiol 1996; 271: H2388–H2398
- Li C, Jackson RM. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol Cell Physiol 2002; 282: C227–C2241
- Pincemail J, Defraigne JO, Detry O, Franssen C, Meurisse M, Limet R. Ischemia-reperfusion injury of rabbit kidney: Comparative effects of desferrioxamine and N-acetylcysteine as antioxidants. Transplant Proc. 2000; 32: 475–476
- Hestin D, Johns EJ. The influence of allopurinol on kidney haemodynamic and excretory responses to renal ischaemia in anaesthetized rats. Br J Pharmacol 1999; 128: 255–261
- Baker GL, Corry RJ, Autor AP. Oxygen free radical induced damage in kidneys subjected to warm ischemia and reperfusion. Protective effect of superoxide dismutase. Ann Surg 1985; 202: 628–641
- Chatterjee PK, Cuzzocrea S, Brown PA, et al. Tempol, a membrane-permeable radical scavenger, reduces oxidant stress-mediated renal dysfunction and injury in the rat. Kidney Int 2000; 58: 658–673
- Bagchi D, Bagchi M, Stohs S, Ray SD, Sen CK, Preuss HG. Cellular protection with proanthocyanidins derived from grape seeds. Ann N Y Acad Sci 2002; 957: 260–270
- Cos P, De Bruyne T, Hermans N, Apers S, Berghe DV, Vlietinck AJ. Proanthocyanidins in health care: Current and new trends. Curr Med Chem 2004; 11: 1345–1359
- Bagchi D, Bagchi M, Stohs SJ, et al. Free radicals and grape seed proanthocyanidin extract: Importance in human health and disease prevention. Toxicology. 2000; 148: 187–197
- Bagchi D, Garg A, Krohn RL, Bagchi M, Tran MX, Stohs SJ. Oxygen free radical scavenging abilities of vitamins C and E, and a grape seed proanthocyanidin extract in vitro. Res Commun Mol Pathol Pharmacol 1997; 95: 179–189
- Chatterjee PK, Patel NS, Kvale EO, et al. Inhibition of inducible nitric oxide synthase reduces renal ischemia/reperfusion injury. Kidney Int 2002; 61: 862–871
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951; 193: 265–275
- Levine RL, Garland D, Oliver CN, et al. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 1990; 186: 464–478
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979; 95: 351–358
- Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem 1988; 34: 497–500
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 1967; 70: 158–169
- Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 2001; 5: 62–71
- Ray SD, Kumar MA, Bagchi D. A novel proanthocyanidin IH636 grape seed extract increases in vivo Bcl-XL expression and prevents acetaminophen-induced programmed and unprogrammed cell death in mouse liver. Arch Biochem Biophys 1999; 369: 42–58
- Roig R, Cascon E, Arola L, Blade C, Salvado MJ. Procyanidins protect Fao cells against hydrogen peroxide-induced oxidative stress. Biochim Biophys Acta 2002; 1572: 25–30
- Arora A, Byrem TM, Nair MG, Strasburg GM. Modulation of liposomal membrane fluidity by flavonoids and isoflavonoids. Arch Biochem Biophys 2000; 373: 102–109
- Moroney MA, Alcaraz MJ, Forder RA, Carey F, Hoult JR. Selectivity of neutrophil 5-lipoxygenase and cyclo-oxygenase inhibition by an anti-inflammatory flavonoid glycoside and related aglycone flavonoids. J Pharm Pharmacol 1988; 40: 787–792
- Morand C, Crespy V, Manach C, Besson C, Demigne C, Remesy C. Plasma metabolites of quercetin and their antioxidant properties. Am J Physiol 1998; 275: R212–R219
- Eraslan G, Saygi S, Essiz D, Aksoy A, Gul H, Macit E. Evaluation of aspect of some oxidative stress parameters using vitamin E, proanthocyanidin and N-acetylcysteine against exposure to cyfluthrin in mice. Pesticide Biochemistry and Physiology 2007; 88: 43–49
- Hammerman C, Goldschmidt D, Caplan MS, et al. Protective effect of bilirubin in ischemia-reperfusion injury in the rat intestine. J Pediatr Gastroenterol Nutr 2002; 35: 344–349
- Ferrer JV, Ariceta J, Guerrero D, et al. Allopurinol and N-acetylcysteine avoid 60% of intestinal necrosis in an ischemia-reperfusion experimental model. Transplant Proc 1998; 30: 2672
- Ozden A, Tetik C, Bilgihan A, et al. Antithrombin III prevents 60 min warm intestinal ischemia reperfusion injury in rats. Res Exp Med (Berl) 1999; 198: 237–246
- Mallick IH, Yang W, Winslet MC, Seifalian AM. Ischemia-reperfusion injury of the intestine and protective strategies against injury. Dig Dis Sci 2004; 49: 1359–1377
- Bagchi D, Ray SD, Patel D, Bagchi M. Protection against drug- and chemical-induced multiorgan toxicity by a novel IH636 grape seed proanthocyanidin extract. Drugs Exp Clin Res 2001; 27: 3–15
- Grzelec-Mojzesowicz M, Sadowski J. Renal tissue NO and intrarenal haemodynamics during experimental variations of NO content in anaesthetised rats. J Physiol Pharmacol 2007; 58: 149–163
- Korkmaz A, Oter S, Sadir S, et al. Peroxynitrite may be involved in bladder damage caused by cyclophosphamide in rats. J Urol 2005; 173: 1793–1796
- Mark LA, Robinson AV, Schulak JA. Inhibition of nitric oxide synthase reduces renal ischemia/reperfusion injury. J Surg Res 2005; 129: 236–241
- Vinas JL, Sola A, Genesca M, Alfaro V, Pi F, Hotter G. NO and NOS isoforms in the development of apoptosis in renal ischemia/reperfusion. Free Radic Biol Med 2006; 40: 992–1003
- Noiri E, Nakao A, Uchida K, et al. Oxidative and nitrosative stress in acute renal ischemia. Am J Physiol Renal Physiol 2001; 281: F948–F957
- Guven A, Uysal B, Akgul O, et al. Scavenging of peroxynitrite reduces renal ischemia/reperfusion injury. Ren Fail 2008; 30(7)747–754
- Arteel GE, Schroeder P, Sies H. Reactions of peroxynitrite with cocoa procyanidin oligomers. J Nutr 2000; 130: 2100S–2104S
- Arteel GE, Sies H. Protection against peroxynitrite by cocoa polyphenol oligomers. FEBS Lett 1999; 462: 167–170