Abstract
Background. Pruritus is a common and bothersome problem in 30–50% of hemodialysis patients. The aim of this study was to determine the effect of gabapentin, 100 mg/three times a week (after each hemodialysis session), on uremic pruritus. Study design. Patients older than 18 years who had undergone hemodialysis for more than three months were enrolled in this double-blind clinical trial. They had experienced pruritus refractory to antihistamines for at least two weeks. The patients were assigned to receive gabapentin 100 mg following hemodialysis for a period of four weeks, and after a washout week, they received the placebo for another four weeks. They were asked to evaluate the severity of their pruritus using a visual analogue scale (VAS). The reduction of pruritus ≥ 50% was accepted as the response. Results. The mean pruritus score reached 6.44 ± 8.4 (p < 0.0001), 15 ± 11.2 (p < 0.001), and 81.11 ± 11.07 (p < 0.001) during gabapentin, washout, and placebo periods, respectively. No significant correlation was found between age, sex, duration of dialysis, underlying diseases, and systolic and diastolic blood pressures and the gabapentin effect. Conclusion. Gabapentin is an effective agent in treating uremic pruritus.
Keywords:
INTRODUCTION
Uremic pruritus is a troubling dermal disorder, secondary to chronic and progressive renal failure; it disturbs patients' sleep and reduces their quality of life.Citation[1],Citation[2] More than half of dialysis patients (hemodialysis or peritoneal dialysis) and about 30% of the patients with acute and chronic renal failure who do not undergo hemodialysis complain about pruritus to some degree.Citation[3]
Pruritus may have peripheral (dermal or neuropathic), central (neuropathic, neurological, or psychogenic), or mixed origins. Uremic pruritus is classified as mixed, and various hypotheses have been formed for justifying its pathogens; accordingly, several treatments with different results were suggested based on the hypotheses. However, none of the treatments managed to cure the very disabling complication.Citation[4],Citation[5]
Gabapentin is an anti-convulsion drug, similar to GABA transmitter in its structure. The effectiveness of gabapentin in controlling convulsions is confirmed by many studies; it has also been demonstrated to be effective in treating chronic neuropathic diseases. The suggested dose for stabilizing the serum level of hemodialysis patients is 200–300 mg following each session of hemodialysis; doses equal to 100 mg, however, are also proposed, aiming at reducing the risk of developing neurotoxicity.Citation[6–8]
Recent studies have suggested that the effectiveness of gabapentin on uremic pruritus by affecting the neural calcium channels.Citation[9],Citation[10]
There are not many studies assessing the effect of gabapentin 100 mg on uremic pruritus. As a result, the purpose of the present study was to examine the effect of gabapentin 100 mg on uremic pruritus of hemodialysis patients.
MATERIALS AND METHODS
This double blind clinical trial was conducted in three hemodialysis centers of Tehran in 2006. Patients with a minimum age of 18 and minimum ESRD duration of three months, suffering from pruritus for at least two weeks, were enrolled in this study. They underwent hemodialysis three times a week for four hours. They had previous attempts of using antihistamines and moisturizers, neither of which was reported to be useful.
The patients had normal hepatic function, and there were no specific underlying diseases intervening with the study. Those with skin lesions, metabolic diseases (producing pruritus), drug allergies, and noncompliant cases were excluded from the study.
After the institutional committee board's approval, informed consent was obtained from all patients. They were asked to quit using anti-pruritus drugs one week prior to the study.
Subsequently, all of the patients underwent a complete neurological examination. The patients were trained to evaluate the severity of their pruritus using a 100 mm visual analogue scale (VAS). Results between 0–25 were considered as low, 25–50 as moderate, 50–75 as high, and 75–100 as severe.
The patients were given gabapentin 100 mg following each session of hemodialysis for four weeks at the beginning of the study. They then received the placebo for another four weeks. One week was considered the washout period between the two treatment phases.
Blood pressure, Kt/V (i.e., the volume of plasma cleared [Kt] divided by the urea distribution volume [V], where K is the dialyzer blood water urea clearance in L per hour, t is dialysis session length in hours, and V is the distribution volume of urea in liters), and other laboratory parameters including Ca, P, Alb, and ipTH were measured at four intervals (i.e., one week prior to the treatment, the drug administration phase, and the washed out, and placebo phases). All samples were taken from the patients before undergoing hemodialysis.
A technician unaware of the phase the patients were in asked them to define the severity of pruritus and the effectiveness of the drug. The median score of pruritus in each interval was considered as the accepted score of pruritus in that period, and the reduction of pruritus for more than 50% was accepted as the positive response.
RESULTS
Thirty-four ESRD patients undergoing hemodialysis were enrolled in this study. There were 8 male (23%) and 26 female (77%) patients. The mean age of the patients was 58.4± 12.5 years ranging between 28 and 73 years. The underlying diseases leading to ESRD in these patients are outlined in .
Table 1 The frequency of the underlying diseases leading to ESRD
Two of the patients discontinued the treatment and were excluded from the study due to representing the drug's side effects (e.g., fatigue, dizziness, and drowsiness). Another patient withdrew from the study, as he did not show any improvement within 10 days after the start of the study. Six other patients were also excluded because of not being cooperative (i.e., poor compliance to the drug). In other words, the complication and no-response rate was calculated to be 8.8% (CI 95% = 0–18.4%).
All patients underwent a complete neurological exam before initiating the treatment; 24 out of the 25 patients who completed the study had a positive neurological exam. All of these 24 cases had signs of distal symmetric polyneuropathy. Hyporeflexia to areflexia was reported in 23 cases. Stocking-glove pattern of hypoesthesia was reported in all of the 24 studied cases, 20 of which had abnormal response in position and vibration tests. Twelve cases had distal motor weakness. Proximal weakness was reported in neither of the cases. Moreover, carpal tunnel syndrome was also reported in one of the cases.
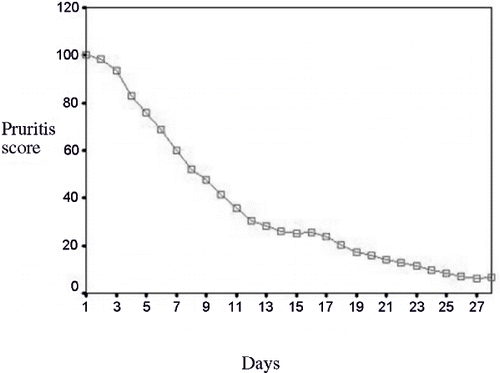
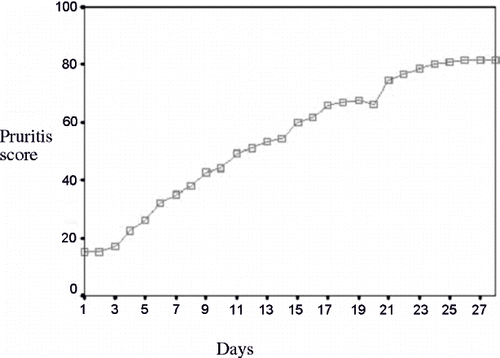
All of the cases eligible for receiving the intervention had the pruritus score of 100 at the beginning of the study. Following the prescription of gabapentin, the score reached 6.44 ± 8.46 (p < 0.001). The mean pruritus score returned to 15 ± 11.27 (p < 0.001) following the one-week washout period. After administering the placebo, the pruritus score was calculated to be 81.88 ± 11.06 (p < 0.001). The pruritus score of the patients during the study period is presented in
Figure 3. The daily mean pruritus score evaluated by the patients in the three phases of the study (i.e., gabapentin therapy, washed out, and placebo).
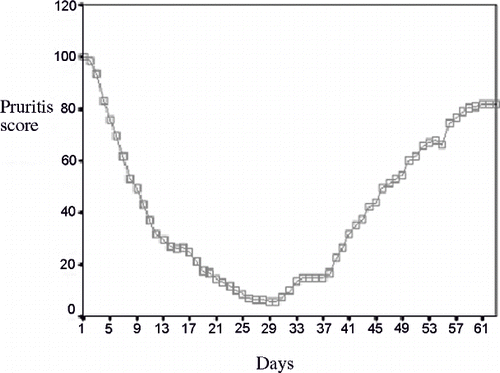
The mean score of pruritus at the end of gabapentin therapy, washout period, and placebo therapy were 6.44 ± 8.46 (p < 0.001), 15 ± 11.27 (p < 0.001), and 81.88 ± 11.06 (p < 0.001), respectively. There was no meaningful relationship between age, gender, the duration of dialysis, the underlying disease leading to ESRD, and KTV and the mean score of pruritus in the three phases of the study.
In the placebo phase, the mean score of pruritus was lower than the score prior to intervention.
The mean score of pruritus in the three phases of gabapentin therapy, washout, and placebo was not correlated with the mean albumin serum (p = 0.84, Pearson correlation = 0.4). Moreover, the mean CRP in the three phases was not correlated with the mean score of pruritus (p = 0.42; Pearson correlation = 0.166).
Some patients claimed that their pruritus was recovered following the treatment of hyperphosphatemia; however, despite the high serum levels of phosphate at the beginning of the study (6.24 ± 2.31), there was no correlation between pruritus and the serum phosphate level. It should be noted that these patients were often treated in order to lower the phosphate level; this treatment did not influence the results of our study.
There was no significant relationship between the patients' age, sex, duration of dialysis, underlying diseases, hemoglobin, Ca, P, Alb, P, ipTH, CRP, systolic and diastolic blood pressures, Kt/V, and the severity of pruritus in the three phases of gabapentin therapy, washout, and placebo therapy. The results are demonstrated in and .
Table 2 Variations of the laboratory variables in different intervention phases
Table 3 The correlation between the variables and their responsiveness to the treatment (reduction in pruritus)
Complications secondary to drug use was reported in two of the total 34 patients enrolled in this study. The complications were limited to dizziness, drowsiness, and unspecified fatigue; however, because the complications emerged by the administration of the drug and disappeared by discontinuing it, the two cases were ethically excluded from the study.
DISCUSSION
The etiology and pathogens of uremic pruritus is not clearly known. Neurological hypothesis,Citation[11],Citation[12] the intervention of the opioid system,Citation[13],Citation[14] the transformed metabolism of bi-valence ions,Citation[15–17] hyperparathyroidism,Citation[18],Citation[19] and the increase of mast cells and etacoidsCitation[18],Citation[20] are the most important mechanisms raised.
Neuropathy is prevalent among uremic patients. More than 65% of the patients with renal failure represent a peripheral nervous disorder,Citation[9] confirming a possible link between the neurologic origins of uremic pruritus.
Conduction disorder in sensory-motor pathways is one of the most prevalent representations in the initial stages of uremia; it is characterized by paresthesia, pricking of the feet, and uneasy foot syndrome.Citation[9]
Pruritus may also be secondary to a reduction in the perception threshold. Nerve root damage may lead to an increased sensitivity to itching stimuli. An abnormal enervation is pointed out in hemodialysis patients.
In the present study, all patients were neurologically examined to compare the treatment results regarding neuropathy findings; but as 24 of the 25 studied patients were reported to have nearly similar evidences of neurological problems, further comparison was not performed. The aforementioned evaluation was not conducted in previous studies.
Similar to the previous studies,Citation[21],Citation[22] there was no significant correlation between pruritus and the demographic factors, Kt/V, the duration of dialysis, and the type of renal dysfunction in the present study.
The results of the present study support the findings of a similar study conducted by Maneti et al. in 2005.Citation[10] In another study carried out on 25 hemodialysis patients, Gunal et al.Citation[9] showed that the mean score of pruritus in the gabapentin phase was significantly different from that of the placebo phase.
We evaluated the relationship of pruritus with inflammatory parameters, albumin as a negative phase protein and CRP as a positive phase protein. Unlike Virga's study,Citation[23] no relation was found between the mean score of pruritus in the three phases of gabapentin therapy, washout, and placebo and the mean serum albumin level in the current study. It can be concluded that albumin is influenced by non-inflammatory factors, including nutritional status as well as the inflammatory factors.Citation[24] In other words, according to that very fact and albumins' relatively long half-life, albumin cannot be considered as a proper criterion for evaluating inflammation.
It is noteworthy that the mean CRP in the three phases was not correlated with the mean score of pruritus. This was similar to the findings of Virga et al.Citation[23]
Several previous studies had considered the resistant and disabling pruritus as a representing sign of hyperthyroidismCitation[20],Citation[21]; however, many others—including the present study—were unable to find any correlation between pruritus and iPTH, Ca, and P.Citation[17],Citation[18] It seems that severe hyperparathyroidism, as the cause of pruritus, emerges in only few patients.
In the present study, there was no relationship between the hemoglobin level of the patients and their pruritus; however, pruritus is reported as the relatively rare complication of iron deficiency anemia.
Despite the fact that Gunal et al.Citation[9] had prescribed higher doses of gabapentin, they did not report any complications; in our study, however, complications were found in two cases. It is possible that the larger sample size or the emotional stress of the patients enrolled in our study had contributed to the higher number of complications. It is noteworthy that these complications were all unspecific and did not cause any problems for the patients.
CONCLUSION
Gabapentin is revealed to be an effective drug in treating uremic pruritus. It is possible that the neurological hypothesis has a significant role in managing uremic pruritus. Also, considering the similar effects of administering gabapentin 100 mg and 300 mg, it is recommended that smaller doses are more cost-beneficial and are probably accompanied by fewer complications in treating these patients; however, further studies are required to approve this hypothesis.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
ACKNOWLEDGMENT
The present study is the report of the research project supported by Tehran University of Medical Sciences and Health Services (Contract No. 2988). We are also indebted to the Urology Research Center, and Research and Development Center of Sina Hospital for their support. We would also like to thank the personnel of the Urology Research Center of Sina Hospital, particularly Ms. Shekarpour and Ms. Heidari. In addition, we appreciate the cooperation of the personnel of the hemodialysis centers of Sina, Imam Khomeini, and Milad hospitals.
REFERENCES
- Lugon JR. Uremic pruritus: A review. Hemodialysis International. 2005; 9: 180–188
- Schwartz IF, Iaina A. Uremic pruritus. Nephrol Dial Transplant. 1999; 14: 834–839
- Urbonas A, et al. Uremic pruritus—an update. Am J Nephrol. 2001; 21: 343–350
- Mettang T, Pauli-Magnus C, Alscher DM. Uremic pruritus—new perspectives and insights from recent trials. Nephrol Dial Transplant. 2002; 17: 1558–1563
- Kimmel M, et al. The role of micro-inflammation in the pathogenesis of uremic pruritus in hemodialysis patients. Nephrol Dial Transplant. 2006; 21: 749–755
- Rose MA, Kam CA. Gabapentin: Pharmacology and its use in pain management. Anaesthesia. 2002; 57: 451–462
- Taylor CP, et al. A summary of mechanistic hypotheses of gabapentin pharmacology. Epilepsy Research. 1998; 29: 233–249
- Bassilios N, Launay-Vacher V, Khoury N, Rondeau E, Deray G, Sraer JD. Gabapentin neurotoxicity in a chronic hemodialysis patient. Nephrol Dial Transplant. 2001; 16: 2112–2113
- Gunal AI, et al. Gabapentin therapy for pruritus in hemodialysis patients: A randomized, placebo-controlled, double-blind trial. Nephrol Dial Transplant. 2004; 19: 3137–3139
- Maneti L, et al. Gabapentin in the treatment of uremic itch: An index case and a pilot evaluation. J Nephrol. 2005; 18: 86–91
- Jedras M, Zakrzewska-Pniewska B, Wardyn K, Switalski M. Uremic neuropathy. II. Is pruritus in dialyzed patients related to neuropathy?. Pol Arch Med Wewn. 1998; 99: 462–469
- Breneman DL, Cardone JS, Blumsack RF, Lather RM, Seaile EA, Pollack VE. Topical capsaicin for the treatment of hemodialysis-related pruritus. J Am Acad Dermatol. 1992; 26: 91–94
- Kumagai H, Utsumi J, Suzuki T, Hayasi M, Saruta T. Endogenous opioid system in uremic pruritus. Abstracts of the Joint Meeting of Seventh World Conference on Clinical Pharmacology and Therapeutics IUPHAR & 4th Congress of the European Association for Clinical Pharmacology and Therapeutics. Br J Clin Pharmacol. 2000; 282
- Okano K, Tagashi Y, Umeuchi H, Ando N, Tanaka T, Kawamura K, Endo T, Kamei J, Nagase H. Anti-pruritic effect of opioid kappa receptor agonist TRK-820. Abstracts of the Joint Meeting of Seventh World Conference on Clinical Pharmacology and Therapeutics IUPHAR & 4th Congress of the European Association for Clinical Pharmacology and Therapeutics. Br J Clin Pharmacol. 2000; 283
- Morton CA, Lafferty M, Hau C, Henderson I, Jones M, Lowe JG. Pruritus and skin hydration during dialysis. Nephrol Dial Transplant. 1996; 11: 2031–2036
- Cho YL, Liu HN, Huang TP, Tarng DC. Uremic pruritus: Roles of parathyroid hormone and substance P. J Am Acad Dermatol. 1997; 36: 538–541
- Friga V, Linos A, Linos D. Is aluminium toxicity responsible for uremic pruritus in chronic hemodialysis patients?. Nephron. 1997; 75: 48–53
- Dimkovia N, Djukanovia L, Radmilovia A, et al. Uremic pruritus and skin mast cells. Nephron. 1992; 61: 5–9
- Mettang T, Fritz P, Weber J, et al. Uremic pruritus in patients on hemodialysis or continuous ambulatory peritoneal dialysis: The role of plasma histamine and skin mast cells. Clin Nephrol. 1990; 34: 136–141
- Ashmore SD, Jones CH, Newstead CG, Daly MJ, Chrystyn H. Ondansetron therapy for uremic pruritus in hemodialysis patients. Am J Kidney Dis. 2000; 35: 827–831
- Mettang T, Fritz P, Weber J. Uremic pruritus in patient on hemodialysis or CAPD: The role of plasma histamine and skin mast cell. Clinical Nephrology. 1990; 34(3)136–141
- Peer G, Kivity S, Agami O, et al. Randomised cross over trial of naltrexone in uremic pruritus. Lancet. 1996; 348: 1552–1554
- Virga G, Visentin I, Lamilia V, et al. Inflammation and pruritus in hemodialysis patients. Nephrol Dial Transplant. 2002; 11: 2164–2169
- Morachiello P, et al. Combined hemodialysis-hemoperfusion in the treatment of secondary hyperparathyroidism of uremic patients. Blood Purif. 1991; 9: 148–152