Abstract
The nephrotoxicity of amikacin (AK) was prevented with pentoxifylline (PTX) in a rat model. Rats were received a single injection of AK (1.2 g/kg, i.p.) with or without PTX pretreatment (25 mg/kg, orally). Renal morphology was investigated by light microscopy. Tissue samples and trunk blood were also obtained to determine renal malondialdehyde (MDA), blood urea nitrogen (BUN), and creatinine (Cr) levels. MDA production was found to be higher in AK group. PTX administration caused a significant decrease in MDA production. Morphological damage in rats given AK was severe in the kidney, whereas in rats given AK plus PTX, no histological changes occurred. It is concluded that PTX could be useful for reducing the nephrotoxic effects of AK.
Keywords:
INTRODUCTION
The clinical use of the chemotherapeutic drug amikacin (AK) is limited due to a dose-dependent nephrotoxicity.Citation[1] Strong evidence supports a role for reactive oxygen species (ROS) in the pathogenesis of AK nephrotoxicity.Citation[2],Citation[3] Recently, pentoxifylline (PTX) has gained considerable interest as a ROS scavenger. Several in vitro studies have confirmed the potential antioxidant effects of this drug.Citation[4–7] To date, there has been no study of the protective effect of PTX, a general phosphodiesterase (PDE) inhibitor, on AK-induced nephrotoxicity. Therefore, we designed this study in rats to investigate the effects of PTX on AK-induced histopathological changes and biological parameters, including malondialdehyde (MDA), blood urea nitrogen (BUN), and serum creatinine (Cr), which are used to monitor the development and extent of renal tubular damage in rats due to oxidative stress.
MATERIALS AND METHODS
Experimental Conditions
Male Wistar rats weighing 200–250 g were placed in a temperature- (21 ± 2°C) and humidity- (60 ± 5%) controlled room in which a 12:12 h light: dark cycle was maintained. The rats were distributed into four groups (six rats in each group):
saline intraperitoneally (i.p.) for three days (control group);
1.2 g/kg AK i.p. (Amikozit 500 mg flk, Eczacibasi Corp, Turkey) at a single doseCitation[3];
25 mg/kg PTXCitation[8] orally (Trental 400 mg draje, Aventis, Turkey) for three days; and
AK i.p. +25 mg/kg PTX orally (Trental 400 mg draje, Aventis, Turkey) one day before AK and continued for two days.
Twenty-four hours after the last PTX, the rats were killed and the kidneys were quickly removed, decapsulated, and divided equally into two longitudinal sections. One half was placed in formaldehyde solution for routine histopathological examination by light microscopy. The other half of the kidney was placed into liquid nitrogen and stored at -70°C until assayed for MDA, a lipid peroxidation product. Trunk blood was extracted to determine the serum levels of BUN and Cr. All experiments in this study were performed in accordance with the guidelines for animal research from the National Institutes of Health and were approved by the Committee on Animal Research at Suleyman Demirel University, Isparta.
Biochemical Determination
A total of 200 mg of kidney tissue were homogenized in ice-cold 150mM KCl for determination of MDA. The MDA content of homogenates was determined spectrophotometrically by measuring the presence of thiobarbituric acid reactive substances. Results are expressed nmol/g tissue. Serum levels of BUN and Cr were determined using the Olympus AutoAnalyzer (Olympus Instruments, Tokyo, Japan). Results are expressed as mg/dL.
Histological Determination
Right kidneys were removed from each rat and divided into the lobes after cleaning. The lobes were fixed in 10% neutral buffered formalin and embedded in paraffin. The paraffin-embedded blocks were cut to 4–5 μm and stained with hematoxylin and eosin (H-E). The slides were then examined using a light microscope (Olympus BX50) and photographed. All histological evaluations were made twice under blind conditions (without knowledge of the treatment).
Statistical Analysis
For statistical analysis, normality was investigated, and it was shown that some values of the parameters did not fit to the normal distribution. Therefore, as stated by Wahhab et al.,Citation[9] considering the small number of cases, non-parametric Kruskal-Wallis test, Mann-Whitney U test, and chi-square test were used to compare groups. Differences were considered as significant for p < 0.05. All results are expressed as mean ± SEM.
RESULTS
Relative to the control group, AK administration to rats increased MDA levels. PTX + AK administration significantly decreased MDA levels. Serum levels of BUN and Cr were changed in the AK-treated animals, but they were not significantly decreased by PTX (see ).
Table 1 The effects of pentoxifylline (PTX) on malondialdehyde (MDA), blood urea nitrogen (BUN), and creatinine (Cr) production in rat kidney with amikacin (AK) treatment
Light microscopic appearance of the renal injury was graded by the criteria defined by Wahhab et al.Citation[9] The light microscopic findings were illustrated in . In control rats, histology of kidney tissues was normal in appearance (see ). Similarly, histological examinations of the PTX group showed no significant differences from the normal histological structure of the kidney tissue (see ). In the AK group (see ), severe hydropic epithelial cell degenerations and moderate tubular dilatation were observed in the most of proximal and distal tubules. Most of the vascular structures were congestive. Additionally, increased connective tissue mass at some areas of interstitium, enlargements in Bowman capsules, sclerosis in some glomerules, mononuclear cell infiltrations, and hemorrhage at some areas of the interstitium were observed at a lesser degree (see ). Some histopathological changes were observed in the PTX + AK group (see ), but to a lesser degree as compared with the AK group. In the PTX + AK group, hydropic epithelial cell degenerations, tubular dilatation, and vascular congestion were observed at minimal levels (see ).
Figure 1. Control group. Kidney tissues were normal in control, H-E × 66. (B): Pentoxyphiline (PTX) group showed no significant differences from the normal histological structure of the kidney tissue, H-E × 66. (C): Amikacin (AK) group. There were significant histopathological changes in renal tissues of AK (), which include hydropic degeneration in proximal and distal tubule epithelial cells, glomerular congestion, sclerosis in some glomerules, tubular dilatations, congestion in peritubular capillaries, hemorrhage in some regions, mononuclear cell infiltrations in parenchyme and connective tissue increment, H-E × 66. (D) AK + PTX group. Kidney tissues were normal in control. H-E × 66.
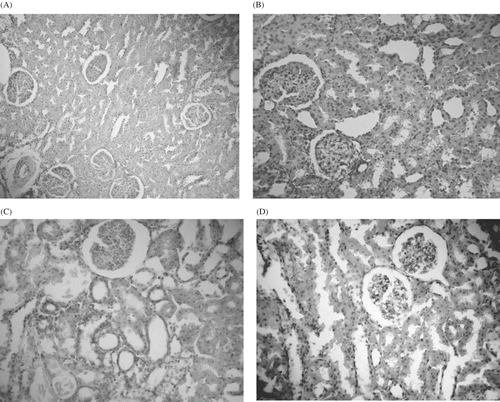
Table 2 Histological changes: ( − ) score (negative score), no structural damage;( + ) score (one positive score), minimal damage; ( ++ ) score (two positive scores), middle damage; ( +++ ) score (three positive scores): severe damage
DISCUSSION
Nephrotoxicity is a major clinical complication of aminoglycoside antibiotics, which are widely used for treatment of gram-negative infectious disease.Citation[1] Drugs with nephrotoxic potential are continuously introduced into pediatric medicine, and assessment of their relative toxicity is important. Aminoglycosides are not metabolized in the body, and the most of injected dose is excreted in the urine, whereas a fraction accumulates in the renal proximal tubules cells, where the concentration of aminoglycosides is several times higher than that in plasma. The concentrated accumulation of aminoglycosides in the proximal tubules cells is associated with its nephrotoxic effects.Citation[10] Nagai et al.Citation[11] also reported that AK accumulated most abundantly in the renal cortex, whereas AK was not detected in the renal papilla, brain, lung, and liver. It is well-established that aminoglycosides accumulated in the renal cortex generate free radicals. Overproduction of free radicals induces an increase in lipid peroxidation (MDA) by destroying unsaturated fatty acids in the cell membrane, causing a decrease in endogenous antioxidants such as glutathione (GSH) in renal tissue.Citation[2] In our study, AK administration to rats increased MDA levels, which are used to monitor the development and extent of renal tubular damage in rats due to oxidative stress. Strong evidence supports a role for reactive oxygen species in the pathogenesis of AK nephrotoxicity, and it was found that antioxidant agents reduced the nephrotoxicity of AK.Citation[2],Citation[3] PTX administration significantly decreased MDA production. In addition, morphological damage in rats given AK was severe in the kidney, whereas in rats given AK plus PTX, no histological changes occurred.
PTX is a non-specific phosphodiesterase (PDE) inhibitor.Citation[4–7] It has been shown that PTX modulates arachidonic acid metabolism, facilitates PGI2 release, hampers the production of various cytokines such as tumor necrosis factor (TNF), and influences the behavior of monocytes, neutrophils, platelets, and endothelial cells in patients with sepsis by inhibiting PDE.Citation[12–16] In animal models, it has been shown that PTX also prevents progressive renal damage associated with septic shock,Citation[17] likely by protecting the renal microcirculation.Citation[12] It may also exert a protective effect on tubular function in patients with ischemic/reperfusion injury,Citation[18] as well as have a protective benefit in nephrotoxicity induced by cisplatin,Citation[19] myoglobinuria,Citation[20] and cyclosporine.Citation[21] A prospective randomized blinded studyCitation[22] in critically ill patients undergoing continuous venovenous hemofiltration (CVVH) revealed that continuous i.v. administration of PTX was successful in blunting the increase in soluble adhesion molecules, which serve as ligands for neutrophils to mount the systemic inflammatory response syndrome. PTX has also been studiedCitation[23] in the prevention of renal insufficiency in elderly patients undergoing cardiac surgery, with positive results.
The superfamily of PDE isozymes consists of at least nine gene families, PDE 1–9.Citation[24] Current evidence indicates that PDE isozymes play a role in several pathobiological processes in kidney cells. Exploration of the PDE isozyme pattern in extracts from nephron segments and cultured renal cells has shown diverse expression of PDE isozymes.Citation[24] The recent development of selective PDE isozyme inhibitors has advanced the identification of the specific functions that are regulated by cyclic adenosine monophosphate-protein kinase A (cAMP-PKA) pathways and linked to a specific PDE isozyme. For example, evidence shows that the PDE 3-linked cAMPPKA pathway suppresses mitogenesis, whereas the PDE 4-linked pathway selectively modulates ROS production in rat mesangial cells, which have been considered to play a central role in the development of glomerulosclerosis.Citation[25] Upon activation of the adjacent cAMP-PKA by selective inhibition of PDE 3, Raf-1 is phosphorylated and 14–3–3 binds, blocking Raf-1 recruitment to the plasma membrane and preventing its activation as well as the downstream mitogenic signal.Citation[25],Citation[26] In contrast, the inhibition of PDE-4 leads to an increase of another cAMP pool and activates adjacent PKA, which subsequently decreases nicotinamide adenine dinucleotide phosphate (NADPH) oxidase assembly and ROS generation via phosphorylating Rap-1a.Citation[27] Inhibitors of PDE 3 and 4 are demonstrated to have a suppressive effect in acute phases or relapses of experimental mesangial proliferative glomerulonephritis.Citation[28] In addition to the suppressive effect on cell proliferation and ROS generation in glomerulonephritis, PDE 4 inhibitors are demonstrated to decrease de novo synthesis and tissue accumulation of the pro-inflammatory cytokines, such as tumour necrosis factora (TNFa), interleukin-1b (IL-1b), IL-6, and interferon (IFNg), which all play important roles in the progression of chronic kidney disease (CKD).Citation[29–31] Furthermore, inhibitors of PDE 3 and 4 have been shown to inhibit fibroblast activation and fibrosis progression.Citation[32],Citation[33] Therefore, various PDE inhibitors, alone or in combination, can target the different pathogenetic mechanisms including cell proliferation, inflammation, as well as extracellular matrix accumulation, and also represent a novel ‘signal transduction pharmacotherapy’ of CKD.
PTX inhibits PDE 1–5 with 50% inhibition (IC50) concentration values ranging between 50 and 200mmol/L.Citation[34],Citation[35] PTX is used clinically to treat patients with peripheral vascular diseases.Citation[36] Notably, PTX is a very safe drug and is usually well tolerated when administered as the conventional controlled release formulation, with gastrointestinal symptoms (i.e., nausea and dyspepsia) and dizziness being the most common complaints in approximately 3% of patients.Citation[36] The primary hemorheologic effects of PTX are caused by increased red blood cell deformability and decreased blood viscosity. The mechanism by which this is achieved has been shown to involve increased erythrocyte adenosine triphosphate (ATP) and other cyclic nucleotide levels. PTX increases intracellular cAMP levels, leading to the inhibition of thromboxane synthesis and an increase of prostacyclin synthesis. Therefore, platelet aggregation and adhesion to vessel walls is inhibited. In addition to this hemorheologically effect, growing evidence has demonstrated that PTX has broad-spectrum renoprotective effects.Citation[37–47]
In conclusion, the significant increase in MDA levels and destructive appearance in histology indicated that AK-induced tissue injury was mediated through oxidative reactions. On the other hand PTX administration protected kidney tissue, very likely by inhibiting PDE and TNFa or other cytokines against the oxidative damage and nephrotoxic effect caused by AK treatment.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Begg EJ, Barclay ML. Aminoglycosides 50 years on. Br J Clin Pharmacol. 1995; 39: 597–603
- Kaynar K, Gul S, Ersoz S, Ozdemir F, Ulusoy H, Ulusoy S. Amikacin-induced nephropathy: Is there any protective way?. Ren Fail. 2007; 29(1)23–27
- Parlakpinar H, Ozer MK, Ucar M, Gaffaroglu M, Vardi N, Koc M, Acet A. Protective effects of caffeic acid phenethyl ester (CAPE) on amikacin-induced nephrotoxicity in rats. Cell Biochem Funct. 2006 Jul–Aug; 24(4)363–367
- Horvath B, Marton Z, Halmosi R, Alexy T, Szapary L, Vekasi J, Biro Z, Habon T, Kesmarky G, Toth K. In vitro antioxidant properties of pentoxifylline, piracetam, and vinpocetine. Clin. Neuropharmacol. 2002; 25(1)37–42
- Bhat VB, Madyastha KM. Antioxidant and radical scavenging properties of 8-oxo derivatives of xanthine drugs pentoxifylline and lisofylline. Biochem. Biophys. Res. Commun. 2001; 288(5)1212–1217
- Freitas JP, Filipe P, Guerra-Rodrigo F. Potential antioxidative effects of pentoxifylline. CR Seances Soc. Biol. Fil. 1995; 189(3)401–405
- Freitas JP, Filipe PM. Pentoxifylline: A hydroxyl radical scavenger. Biol. Trace Elem. Res. 1995; 47(1–3)307–311
- de Campos T, Deree J, Martins JO, Loomis WH, Shenvi E, Putnam JG, Coimbra R. Pentoxifylline attenuates pulmonary inflammation and neutrophil activation in experimental acute pancreatitis. Pancreas. 2008 Jul; 37(1)42–49
- Wahhab MA, Nada SA, Arbid MS. Ochratoxicosis: Prevention of developmental toxicity by L-methionine in rats. J Applied Toxicol. 1999; 19: 7–12
- Humes HD. Aminoglycoside nephrotoxicity. Kidney Int. 1988; 33: 900–911
- Nagai J, Tanaka H, Nakanishi N, Murakami T, Takano M. Role of megalin in renal handling of aminoglycosides. Am J Physiol Renal Physiol. 2001; 28: 337–344
- Krysztopik RJ, Matheson PJ, Spain DA, et al. Lazaroid and pentoxifylline suppress sepsis-induced increases in renal vascular resistance via altered arachidonic acid metabolism. J Surg Res. 2000; 93: 75–81
- Ambrus JL, Halpern J, Mahafzah M, et al. Platelet aggregation in septic shock: Effects of pentoxifylline. J Med. 1990; 21: 121–128
- Fink MP. Whither pentoxifylline?. Crit Care Med. 1999; 27: 19–20
- Ward A, Clissold SP. Pentoxifylline: A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic efficacy. Drugs. 1987; 34: 50–97
- Mandell GL. ARDS, neutrophils, and pentoxifylline. Am Rev Respir Dis. 1988; 138: 1103–1105
- Berens KL, Langston JD, Wasan KM, et al. Influence of pentoxifylline and related analogues in endotoxemic renal failure. Circ Shock. 1991; 34: 344–348
- Kim YK, Yoo JH, Woo JS, et al. Effect of pentoxifylline on ischemic acute renal failure in rabbits. Ren Fail. 2001; 23: 757–772
- Kim YK, Choi TR, Kwon CH, et al. Beneficial effect of pentoxifylline on cisplatin-induced acute renal failure in rabbits. Ren Fail. 2003; 25: 909–922
- Savic V, Vlahovic P, Djordjevic V, et al. Nephroprotective effects of pentoxifylline in experimental myoglobinuric acute renal failure. Pathol Biol. (Paris). 2002; 50: 599–607
- Shifow AA, Naidu MU, Kumar KV, et al. Effect of pentoxifylline on cyclosporine-induced nephrotoxicity in rats. Indian J Exp Biol. 2000; 38: 347–352
- Boldt J, Muller M, Heesen M, al et. The effects of pentoxifylline on circulating adhesion molecules in critically ill patients with ARF treated by CVVH. Intensive Care Med. 1996; 22: 305–311
- Boldt J, Brosch C, Piper SN, et al. Influence of prophylactic use of pentoxifylline on postoperative organ function in elderly cardiac surgery patients. Crit Care Med. 2001; 29: 952–958
- Dousa TP. Cyclic-3’, 5’-nucleotide phosphodiesterase isozymes in cell biology and pathophysiology of the kidney. Kidney Int. 1999; 55: 29–62
- Dousa TP. Signaling role of PDE isozymes in pathobiology of glomerular mesangial cells. Studies in vitro and in vivo. Cell Biochem. Biophys. 1998; 29: 19–34
- Dumaz N, Marais R. Protein kinase A blocks Raf-1 activity by stimulating 14–3-3 binding and blocking Raf-1 interaction with Ras. J. Biol. Chem. 2003; 278(29)819–823
- Chini CS, Chini EN, Williams JM, Matousovic K, Dousa TP. Formation of reactive oxygen metabolites in glomeruli is suppressed by inhibition of cAMP phosphodiesterase isozyme type IV. Kidney Int. 1994; 46: 28–36
- Tsuboi Y, Shankland SJ, Grande JP, Walker HJ, Johnson RJ, Dousa TP. Suppression of mesangial proliferative glomerulonephritis development in rats by inhibitors of cAMP phosphodiesterase isozymes types III and IV. J. Clin. Invest. 1996; 98: 262–270
- Seldon PM, Barnes PJ, Meja K, Giembycz MA. Suppression of lipopolysaccharide-induced tumour necrosis factor-a generation from human peripheral blood monocytes by inhibitors of phosphodiesterase 4: Interaction with stimulants of adenylyl cyclase. Mol. Pharmacol. 1995; 48: 747–757
- Klahr S, Morrissey J. Progression of chronic renal disease. Am. J. Kidney Dis. 2003; 41: S3–S7
- Heystek HC, Thierry AC, Soulard P, Moulon C. Phosphodiesterase 4 inhibitors reduce human dendritic cell inflammatory cytokine production and Th1-polarizing capacity. Int. Immunol. 2003; 15: 827–835
- Shimizu E, Kobayashi Y, Oki Y, Kawasaki T, Yoshimi T, Nakamura H. OPC-13013, a cyclic nucleotide phosphodiesterase type III inhibitor, inhibits cell proliferation and transdifferentiation of cultured rat hepatic stellate cells. Life. Sci. 1999; 64: 2081–2088
- Kohyama T, Liu X, Wen FQ, et al. PDE4 inhibitors attenuate fibroblast chemotaxis and contraction of native collagen gels. Am. J. Respir. Cell. Mol. Biol. 2002; 26: 694–701
- Meskini N, Nemoz G, Okyayuz-Baklouti I, Lagarde M, Prigent AF. Phosphodiesterase inhibitory profile of some related xanthine derivatives pharmacologically active on the peripheral microcirculation. Biochem. Pharmacol. 1994; 47: 781–788
- Schermuly RT, Roehl A, Weissmann N, et al. Combination of nonspecific PDE inhibitors with inhaled prostacyclin in experimental pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001; 281: L1361–L1368
- Ward A, Clissold SI. Pentoxifylline: A review of its pharmacodynamic and pharmacokinetic properties and its therapeutic efficacy. Drugs. 1987; 34: 50–97
- Lin SL, Chen YM, Chien CT, Chiang WC, Tsai CC, Tsai TJ. Pentoxifylline attenuated the renal disease progression in rats with remnant kidney. J. Am. Soc. Nephrol. 2002; 13: 2916–2929
- Tsai TJ, Lin RH, Chang CC, et al. Vasodilator agents modulate rat glomerular mesangial cell growth and collagen synthesis. Nephron. 1995; 70: 91–100
- Albornoz LE, Sanchez SB, Bandi JC, Canteros G, de las Heras M, Mastai RC. Pentoxifylline reduces nephrotoxicity associated with cyclosporine in the rat by its rheological properties. Transplant. 1997; 64: 1404–1407
- Chen YM, Chien CT, Hu-Tsai MI, et al. Pentoxifylline attenuates experimental mesangial proliferative glomerulonephritis. Kidney Int. 1999; 56: 932–943
- Lin SL, Chen RH, Chen YM, Chiang WC, Tsai TJ, Hsieh BS. Pentoxifylline inhibits platelet-derived growth factor-stimulated cyclin D1 expression in mesangial cells by blocking Akt membrane translocation. Mol. Pharmacol. 2003; 64: 811–822
- Chen YM, Chiang WC, Lin SL, Wu KD, Tsai TJ, Hsieh BS. Dual regulation of TNF-a-induced CCL2/monocyte chemoattractant protein-1 expression in vascular smooth muscle sells by NF-kB and AP-1: Modulation by type III phosphodiesterase inhibition. J. Pharmacol. Exp. Ther. 2004; 309: 978–986
- Chen YM, Ng YY, Lin SL, Chiang WC, Lan HY, Tsai TJ. Pentoxifylline suppresses renal tumour necrosis factor-a and ameliorates experimental crescentic glomerulonephritis in rats. Nephrol. Dial. Transplant. 2004; 19: 1106–1115
- Navarro JF, Mora C, Rivero A, et al. Urinary protein excretion and serum tumour necrosis factor in diabetic patients with advanced renal failure: Effects of pentoxifylline administration. Am. J. Kidney. Dis. 1999; 33: 458–463
- Navarro JF, Mora C, Muros M, Maca M, Garca J. Effects of pentoxifylline administration on urinary N-acetyl-b- glucosaminidase excretion in type 2 diabetic patients: A short-term, prospective randomized study. Am. J. Kidney Dis. 2003; 42: 264–270
- Ducloux D, Bresson-Vautrin C, Chalopin JM. Use of pentoxifylline in membranous nephropathy. Lancet. 2001; 357: 1672–1673
- Segal R, Dayan M, Zinger H, Mozes E. Suppression of experimental systemic lupus erythematosus (SLE) in mice via TNF inhibition by an anti-TNF-a monoclonal antibody and by pentoxifylline. Lupus. 2001; 10: 23–31