Abstract
Kikuchi-Fujimoto disease (KFD) is a benign and self-limited disease of unknown etiology that affects mainly young women. It presents with localized lymphadenopathy, usually cervical, accompanied with fever, night sweats, and leucopenia. KFD has been rarely described in association with autoimmune disorders, mainly systemic lupus erythematosus (SLE). We report the case of a young patient presenting with KFD coinciding with SLE. The association of KFD and SLE is reviewed. Moreover, a possible pathogenetic role of Ebstein-Barr virus linking the two clinical entities is discussed.
INTRODUCTION
Kikuchi-Fujimoto lymphadenopathy or disease (KFD) is a benign and self-limited disease of unknown etiology that presents with localized lymphadenopathy, usually cervical, accompanied with fever, night sweats, and leucopenia. KFD is an extremely rare disease known to have a worldwide distribution with a higher prevalence among Japanese and other Asiatic individuals. It has been rarely described in association with autoimmune disorders, mainly systemic lupus erythematosus (SLE), and its diagnosis can precede, postdate, or coincide with the diagnosis of SLE.
We report the case of a young patient presenting with fever, leucopenia, and lymphadenopathy, histologically diagnosed as KFD. The patient's condition deteriorated, and SLE was subsequently diagnosed. The association of KFD and SLE is reviewed. Moreover, a possible pathogenetic role of Ebstein-Barr virus linking the two clinical entities is discussed.
CASE REPORT
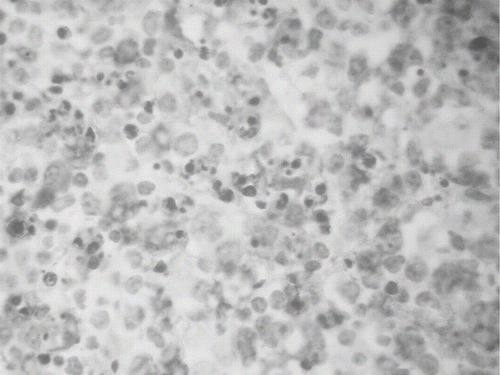
A 23-year-old female patient was initially admitted to the Hematology Department due to fever, chills, and cervical lymphadenopathy. Laboratory tests revealed leucopenia, anemia, elevated ESR and CRP, and mild renal dysfunction with active urinary sediment (see ). Blood and urinary cultures and mucous smears were negative for bacteria. A myelogram was unremarkable. Enhanced CT scanning revealed cervical and mediastinal lymphadenopathy. A fine needle aspiration biopsy of an affected lymph node showed lesions compatible with Kikuchi-Fujimoto disease (see and ). There were clusters of histiocytes and modified lymphocytes surrounding marked areas of tissue necrosis, mild infiltrations of plasmacytes, and abundant nuclear debris. In the remaining lymphoid tissue, mature lymphocytes with scattered histiocytes, plasmacytes, and immunoblasts can be seen. Immunohistochemistry revealed necrotic areas surrounded by modified lymphocytes of T-cell origin, predominantly CD8+ and just very few CD4+ cells, while the small mature lymphocytes were CD20+. The histiocytes stained positively for CD68.
Table 1 Laboratory results on admission and the fifth day
Figure 1. Cervical lymph node showing areas of tissue necrosis with extensive apoptosis and abundant nuclear debris but no leukocytic infiltration (H+E stain).

Figure 2. Prominent histiocytic proliferation in cervical lymph node (positive CD68 expression-peroxidase stain).
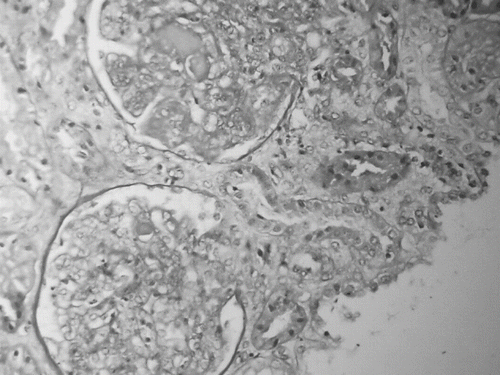
Under the diagnosis of KFD, treatment with steroids was initiated and resulted in prompt defeverness. However, renal function parameters deteriorated and renal consultation was requested. The patient underwent an ultrasound-guided kidney biopsy (see ). Renal histology showed marked global endocapillary and mesangial proliferation with widespread polymorphonuclear infiltrations. Several capillaries contained eosinophilic intraluminal deposits that formed hyaline thrombi. Occasional synechiae with Bowman's capsule were observed. Inflammatory infiltrates were dispersed in the interstitium, while proteinaceous casts could be seen in the tubular lumen. Immunofluorescence confirmed the diagnosis of type IV SLE. At the same time, highly positive titers of antinuclear, anti-DNA, anti-SSA, anti-SSB, and anti-RNP antibodies came back, along with very low levels of C3 and C4. Moreover, high IgG and IgM titers of Ebstein-Barr virus (EBV) were noted, and the presence of EBV was confirmed with PCR.
Figure 3. Renal tissue section showing marked global endocapillary and mesangial proliferation with scattered polymorphonuclear infiltrates. Several capillaries contain eosinophilic intraluminal deposits that form hyaline thrombi (H+E stain).
The patient's condition deteriorated, despite aggressive immunosuppressive treatment with high dose corticosteroids and pulse cyclophosphamide and antiviral treatment. She developed thrombotic thrombocytopenic purpura and pulmonary edema refractory to standard medication. A series of plasmapheresis treatments combined with dialysis were initiated in order to control and stabilize the clinical status of the patient, though with limited efficacy. Ultimately, her disease responded to the administration of rituximab 375 mg/m2 once a week for four weeks. Currently, the patient's condition is stable with renal function parameters within normal range under low dose corticosteroids and p.o. cyclophosphamide.
DISCUSSION
Kikuchi-Fujimoto lymphadenopathy or disease (KFD) or histiocytic necrotizing lymphadenitis is an enigmatic, benign, and self-limiting syndrome. It is often characterized by regional lymphadenopathy with tenderness and usually accompanied by mild fever and night sweats. Initially described in 1972 in Japan, KFD has a worldwide distribution with a higher prevalence among Japanese and Asiatic individuals.Citation[1] Affected patients are most often female adults younger than 40 years (female/male ratio, 4:1). The onset is acute or subacute, evolving during a period of 2–3 weeks. Cervical lymphadenopathy is present in 56–98%, although nearly all lymph nodes can be affected (mediastinal, peritoneal and retroperitoneal regions are rarely involved). In addition, patients with KFD present with fever, usually low-grade, and less frequently with night sweats, weight loss, and arthritis. Laboratory wise, leucopenia, increased erythrocyte sedimentation rate and anemia were the most common findings. Involvement of extranodal sites is uncommon, but skin, mucosal, and bone marrow involvement and liver dysfunction have been reported.Citation[1–4]
The etiology of KFD is a matter of much speculation.Citation[1–3] A viral or autoimmune cause has been suggested. The role of Ebstein-Barr virus (EBV), as well as other viruses (HHV 6, HHV 8, parvovirus B19) in the pathogenesis of KFD remains controversial and not convincingly demonstrated.Citation[1],Citation[3],Citation[5] A viral infection is possible nonetheless by virtue of clinical manifestations, as described by Unger et al.,Citation[6] including upper respiratory prodrome, atypical lymphocytosis, and lack of response to antibiotic therapy and certain histopathologic features (i.e., T cells as revealed by immunological markers). Two pathological studies from Korea found no evidence that EBV plays any role in the pathogenesis of KFD, as they failed to detect any viral DNA in the cases analyzed.Citation[7],Citation[8] The same conclusion is reached by Hollinsworth et al.,Citation[9] although in their study EBV DNA was detected by the polymerase chain reaction and in situ hybridization in 40% of the patients, with an additional 15% giving a weaker positive signal. Sumiyoshi et al.Citation[10] examined the cervical lymph nodes of 30 patients with KFD. The nodes of 16 of their patients showed amplified EBV DNA by PCR. Our patient presented with high titers of both IgG and IgM for EBV and a highly positive signal for EBV DNA with PCR.
KFD generally is diagnosed on the basis of an excisional biopsy of affected lymph nodes. The characteristic histopathologic findings include irregular paracortical areas of coagulative necrosis with abundant karyorrhectic debris, which can distort the nodal architecture, and large numbers of different types of histiocytes at the margin of the necrotic areas. The karyorrhectic foci are formed by different cellular types, predominantly histiocytes and plasmatoid monocytes but also immunoblasts, and small and large lymphocytes may be found. The immunophenotype of KFD consists of a predominance of T cells, with very few B cells. There is a predominance of CD8+ cells over CD4+ cells, along with a decreased ratio in the affected areas of the lymph node. The histiocytes express histiocyte-associated antigens, such as lysozyme, myeloperoxidase, and CD68.Citation[1],Citation[4],Citation[5]
Several articles have described an association of KFD with autoimmune disorders, mainly SLE, mixed connective tissue disease, anti-phospholipid syndrome, thyroiditis, polymyositis, scleroderma, autoimmune hepatitis, Still's disease, as well as various other diseases (e.g., viral diseases, tuberculosis, lymphomas, hemophagocytic syndrome). The strongest link seems to be with SLE,Citation[1],Citation[3] although the exact nature of this association has not yet been established. Santana et al.Citation[11] searched the literature in 2003 and reported 35 case of KFD with SLE distributed all over the world. SLE was diagnosed prior to KFD in seven cases, simultaneously in 14 cases, and afterward in 14 cases. Kukukardali et al.Citation[12] analyzed 244 cases of KFD and found an association with SLE in 32 cases (13%). It is believed that a pathogenic link between KFD and SLE exists. It has been hypothesized that KFD could represent a self-limited lupus-like condition induced by activated lymphocytes; however, the nature of this link remains to be determined.Citation[13] On the other hand, SLE has also been associated with viral infections, most interesting in regard to our case regarding the EBV. It has been shown that patients with SLE have abnormally elevated EBV load in their blood. Uk-Yeol et al.Citation[14] and Gross et al.Citation[15] discovered an abnormally increased proportion of EBV-infected B cells in SLE patients and suggested that this may contribute to enhanced antibody production. This abnormally high frequency of EBV-infected cells has been associated with SLE disease flares. A defective control of latent EBV infection in SLE patients has been shown that leads to a ∼40-fold increase of EBV viral loads compared to controls, a finding not explained by disease activity or immunosuppressive medication.Citation[16] This has been attributed probably to altered T cell responses against EBV.Citation[16],Citation[17] It has been demonstrated that the Epstein-Barr virus Nuclear Antigen-1 (EBNA-1) elicits the production of IgG antibodies to Sm and to double-stranded DNA.Citation[18] Harley et al.Citation[19] found that the first lupus-specific autoantibodies are anti-Sm and anti-60 KD Ro and arise from particular autoantibodies against EBNA-1. The above researchers suggest that an EBV infection generates anti-EBNA-1, followed by those particular anti-EBNA-1 antibodies that also bind to lupus-specific autoantigens (Sm or Ro), leading to the development of more complex autoimmune response and, finally, culminating in clinical disease. Hence they regard EBV as an environmental risk factor for lupus.Citation[19]
Patients with KFD present with elevation of 2'-5'-oligoadenylate synthetase in the white blood cells and tubuloreticular structures in the histiocytes, activated lymphocytes, and endothelial cells.Citation[13–20] These findings, strongly suggestive of hyperimmune response of viral etiology, have also been demonstrated in endothelial cells or lymphocytes of patients with SLE and other autoimmune disorders.Citation[21] In conclusion, all of the above-mentioned data prompted us to hypothesize first that KFD and SLE could be two sides from the same coin of autoimmune disorders, the first being a milder manifestation of the latter, or two distinct autoimmune entities linked by the same pathogenetic factor, namely EBV infection.
In summary, we describe the case of a young woman presenting with Kikuchi-Fujimoto disease with co-existing systemic lupus erythematosus, possibly following an Epstein-Barr infection.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Bosch X, Guilabert A, Miquel R, Campo E. Enigmatic Kikuchi-Fujimoto disease: A comprehensive review. Am J Clin Pathol. 2004; 122: 141–152
- Poulose V, Chiam P, Poh WT. Kikuchi's disease: A Singapore case series. Singapore Med J. 2005; 46(5)229–232
- Dorfman RF, Berry GJ. Kikuchi's histiocytic necrotizing lymphadenitis: An analysis of 108 cases with emphasis on differential diagnosis. Semin Diagn Pathol. 1988; 5: 329–345
- Tsang WYW, Chan JKC, Ng CS. Kikuchi's lymphadenitis: A morphologic analysis of 75 cases with special reference to unusual features. Am J Surg Pathol. 1994; 18: 219–231
- Kuo T. Kikuchi's disease (histiocytic necrotizing lymphadenitis): A clinicopathologic study of 79 cases with analysis of histologic subtypes, immunohistology, and DNA ploidy. Am J Surg Pathol. 1995; 19: 798–809
- Unger PD, Rappaport KM, Strauchen JA. Necrotizing lymphadenitis (Kikuchi's disease). Report of four cases of an unusual pseudo-lymphomatous lesion and immunologic marker studies. Arch Pathol Lab Med. 1987; 111: 1031–1034
- Kyung-Ja C, Seung-Sook L, Shin-Kwang K. Histiocytic necrotizing lymphadenitis: A clinico-pathologic study of 45 cases with in situ hybridization for Epstei-Barr virus and hepatitis-B virus. J Kor Med Sci. 1996; 11(5)409–414
- Jooryung H, Hyun-Sook C, Sung-Sook K, Gyungyub G. A study of the viral etiology of histiocytic necrotizing lymphadenitis (Kikuchi-Fujimoto disease). J Kor Med Sci. 1998; 13: 27–30
- Hollingsworth HC, Peiper SC, Weiss LM, Raffeld M, Jaffe ES. An investigation of the viral pathogenesis of Kikuchi-Fujimoto disease. Lack of evidence for Epstein-Barr virus or human herpesvirus type 6 as the causative agents. Arch Pathol Lab Med. 1994; 118(2)134–140
- Sumiyoshi Y, Kikuchi M, Minematu T, et al. Analysis of herpesvirus genome in Kikuchi's disease. Virchows Arch. 1994; 424(4)437–440
- Santana A, Lessa B, Glarao L, Lima I, Santiago M. Kikuchi-Fujimoto's disease associated with systemic lupus erythematosus: Case report and review of the literature. Clin Rheumatol. 2005; 24(1)60–63
- Kucukardali Y, Solmazgul E, Kunter E, Oncul O, Yildirim S, Kaplan M. Kikuchi-Fujimoto disease: analysis of 244 cases. Clin Rheumatol. 2007; 26(1)50–54
- Correa H. Kikuchi-Fujimoto disease: An exuberant localized T cell activation arrested by histiocytes?. Medscape General Medicine 1999; 1(3)5
- Uk-Yeol M, Su-Jin P, Sang-Taek O, et al. Patients with systemic lupus erythematosus have abnormally elevated Epstein-Barr virus load in blood. Arthritis Res Ther. 2004; 6: R295–R302
- Gross A, Hochberg D, Rand W, Thorley-Lawson D. EBV and systemic lupus erythematosus: A new perspective. J Immunol. 2005; 174: 6599–6607
- Kang I, Quan T, Nolasco H, et al. Defective control of latent Epstein-Barr virus infection in systemic lupus erythematosus. J Immunol. 2004; 172: 1287–1294
- Berner BR, Tary-Lehmann M, Yonkers NL, et al. Phenotypic and functional analysis of EBV-specific memory CD8 cells in SLE. Cell Immunol. 2005; 235(1)29–38
- Sundar K, Jacques S, Gottlieb P, et al. Expression of the Epstein-Barr virus nuclear antigen-1 (EBNA-1) in the mouse can elicit the production of anti-dsDNA and anti-Sm antibodies. J Autoimmun. 2004; 23(2)127–140
- Harley JB, James JA. Epstein-Barr virus infection induces lupus autoimmunity. Bulletin of the NYU Hospital for Joint Diseases. 2006; 64(1)45–49
- Imamura M, Ueno H, Matsuura A, et al. An ultrastructural study of subacute necrotizing lymphadenitis. Am J Surg Pathol. 1982; 107: 292–299
- Haas JE, Yunis EJ. Tubular inclusions of systemic lupus erythematosus. Ultrastructural observations regarding their possible viral nature. Exp Mol Pathol. 1970; 12: 257–263
