Abstract
5-aminosalicylate compounds (mesalazine, sulfasalazine) are widely used in therapy of inflammatory bowel diseases. Mesalazine-induced interstitial nephritis is a rare complication; however, the morbidity in an affected individual is high. Regular renal screening in patients treated with 5-aminosalicylate compounds is important. A 15-year-old boy with treated idiopathic proctocolitis, consequent mesalazine-induced nephritis, and a favorable response to corticotherapy is presented.
INTRODUCTION
5-aminosalicylate (5-ASA) compounds (mesalazine, sulfasalazine) are widely used in therapy of inflammatory bowel diseases (IBD).Citation[1],Citation[2] Mesalazine-induced interstitial nephritis is a rare complication that occurs in fewer than 1 out of 500 treated patients. This complication shows a male predominance, an absence of specific symptoms or findings in urinalysis, a high frequency of chronic renal insufficiency and a favorable response of active nephritis to steroid therapy.Citation[1–6]
CASE REPORT
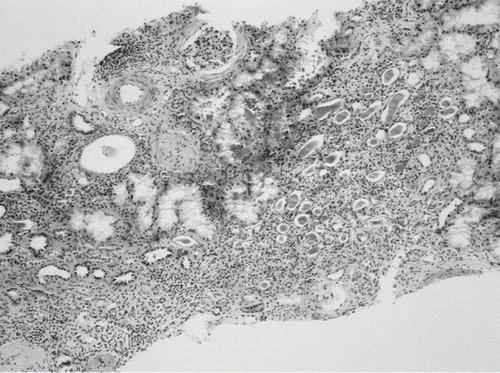
A 15-year-old boy with idiopathic proctocolitis has been treated since eleven years of age. He received sulfasalazine for one year, after which the treatment had been changed to mesalazine in dose of 3 g daily, azathioprine with maximal daily dose 3 mg/kg body weight, and prednisolone in tapering daily dose to 8 mg/kg. Upon regular examination at the age of 15 years, he had no complaints, but there was weight loss of 4.5 kg during the previous four months. Among laboratory findings, there was high erythrocyte sedimentation rate (ESR) 60/88, anemia with hemoglobin level 93g/L (normal 130–180 g/L), elevated level of blood urea nitrogen (10.2 mmol/L; normal 2.9–8.9 mmol/L), and borderline creatinine (126 μmol/L; normal 53–133 μmol/L). There was no improvement in laboratory findings after cessation of mesalazine therapy, as fever and erythema nodosum developed within two weeks. One month later, his height was 167 cm (−1.0 SD), weight 46.5 kg (−1.6 SD), BMI 16.7 (−1.5 SD), and blood pressure 120/70 mm Hg; he was pale without evidence of edema and with erythema nodosum on his legs. Laboratory assessment revealed high ESR 90/130, low hemoglobin level 93 g/L, normal blood urea nitrogen 6.5 mmol/L, and borderline creatinine 130 μmol/L. Trace protein was present in urine, and microscopic urinalysis was overall normal, with the exception of leukocyturia in one urine specimen. 24-hour proteinuria was maximally 0.19 g per day with tubular pattern, creatinine clearance was low (1.036 mL/s/1.73 m2; normal 1.61–2.42 mL/s/1.73 m2), and concentration ability of kidney after desmopressin was 838 mmol/kg. Renal ultrasonography and voiding cystourethrography (VCUG) were normal. Renal scintigraphy with 99mTc-dimercaptosuccinic acid (DMSA scan) showed bilateral diffuse non-homogenic pattern with multifocal defects of isotope uptake. Kidney biopsy revealed chronic tubulointerstitial involvement with sclerosed majority of glomeruli (see ). Based on the combination of mesalazine treatment, borderline serum creatinine level, decreased creatinine clearance and histologic finding of tubulointerstitial nephritis in the absence of any other disease, the diagnosis of mesalazine-induced nephritis was established. We started methylprednisolone pulse therapy (intravenous methylprednisolone 1000 mg/day in three consecutive days) with subsequent oral prednisone treatment (60 mg/day). Remarkable improvement in laboratory values (ESR 16/36, S-creatinine 97 μmol/, creatinine clearance 1.45 mL/s/1.73 m2) was observed after 21 days of treatment.
DISCUSSION
Mesalazine is a widely used drug in pediatric IBD, and is effective as primary treatment and maintenance therapy. It is usually well tolerated.Citation[1],Citation[2],Citation[4] However, several studies suggest an association between the use 5-ASA in patients with IBD and the development of chronic tubulo-interstitial nephritis.Citation[1–6],Citation[8–10] Apart from lesions associated with 5-ASA treatment, the IBD itself may also induce renal impairment.Citation[4],Citation[7] Nephrotoxicity is exceptional (mean rate of only 0.26% per patient-year) in 5-ASA treated patients.Citation[1] While the incidence of this adverse event in the population of patients with IBD treated with mesalazine is low,Citation[4],Citation[6] the morbidity in an affected individual is high, as there is 61% frequency of residual chronic renal insufficiency with 13% of patients developing end-stage renal disease.Citation[3] The absence of a clear relationship between 5-ASA dose and the risk of nephrotoxicity suggests that this complication is idiosyncratic rather than dose-related.Citation[1] Most of the patients with renal disease associated with 5-ASA treatment suffered interstitial nephritis, with symptoms and signs being nonspecific, which may delay detection for many months. The nephrotoxicity potential of mesalazine and sulfasalazine seems to be similar.Citation[1] Routine monitoring of renal function is simple and inexpensive and could prevent this outcome.Citation[1–3],Citation[5],Citation[6],Citation[8–10] The optimal monitoring schedule of serum creatinine in patients receiving 5-ASA treatment remains to be established, as there is no evidence to date that either the test or the frequency of testing improves patient outcomes. Based on the available data, serum creatinine should be estimated prior to commencing treatment and monthly for the first three months, then every three months for the next nine months, every six months thereafter, and annually after five years of treatment.Citation[6],Citation[8]
Mesalazine should be withdrawn when renal impairment manifests in a patient with IBD; if this does not result in a fall in serum creatinine, then renal biopsy should be considered. A trial of high-dose steroid may be recommended in patients whose renal function does not respond to drug withdrawal,Citation[1] as corticotherapy was shown to have a favorable outcome.Citation[1],Citation[3],Citation[8–10] The personal history and clinical findings including histology results of renal biopsy in our patient were indicative of mesalazine-induced nephritis. The corticotherapy resulted in overall improvement of both clinical state and laboratory markers. Despite the clinical and laboratory improvement, the overall prognosis of renal function in this boy remains uncertain.
CONCLUSIONS
We report a rare complication of mesalazine treatment. As demonstrated in this case, regular renal screening in patients treated with 5-aminosalicylate compounds is important. Serum creatinine elevation is frequently the only but late sign of mesalazine-induced interstitial nephritis.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Gisbert JP, González-Lama Y, Maté J. 5-Aminosalicylates and renal function in inflammatory bowel disease: A systematic review. Inflamm Bowel Dis. 2007; 13: 629–638
- Uslu N, Demir H, Saltik-Temizel IN, et al. Acute tubular injury associated with mesalazine therapy in an adolescent girl with inflammatory bowel disease. Dig Dis Sci. 2007; 52: 2926–2929
- Arend LJ, Springate JE. Interstitial nephritis from mesalazine: Case report and literature review. Pediatr Nephrol. 2004; 19: 550–553
- Elseviers MM, D'Haens G, Lerebours E, et al. Renal impairment in patients with inflammatory bowel disease: Association with aminosalicylate therapy?. Clin Nephrol. 2004; 61: 83–89
- World MJ, Stevens PE, Ashton MA, et al. Mesalazine-associated interstitial nephritis. Nephrol Dial Transplant. 1996; 11: 614–621
- Corrigan G, Stevens PE. Review article: interstitial nephritis associated with the use of mesalazine in inflammatory bowel disease. Aliment Pharmacol Ther. 2000; 14: 1–6
- Benador N, Grimm P, Lemire J, et al. Interstitial nephritis in children with Crohn's disease. Clin Pediatr. 2000; 39: 253–254
- Van Biervliet S, Raes A, Vande Walle J, et al. Mesalazine interstitial nephritis presenting as colitis ulcerosa exacerbation. Acta Gastroenterol Belg. 2006; 69: 321–322
- Tadic M, Grgurevic I, Scukanec-Spoljar M, et al. Acute interstitial nephritis due to mesalazine. Nephrology. 2005; 10: 103–105
- Ohya M, Otani H, Kimura K, et al. Interstitial nephritis induced by mesalazine. Nippon Jinzo Gakkai Shi. 2002; 44: 414–419