Abstract
Peripartum acute renal failure is an important complication related to pregnancy leading to significant morbidity and mortality. Emphysematous pyelonephritis (EPN) is a severe necrotizing infection of the renal parenchyma, with formation of gas within the collecting system, renal parenchyma, or perirenal tissues. EPN is common in persons with diabetes or urinary tract obstruction. Herein we report a case of bilateral emphysematous pyelonephritis in a postpartum lady who had no evidence of diabetes or urinary tract obstruction. Management of this condition has traditionally been aggressive, and surgery has been considered mandatory. Our patient was managed successfully with antibiotics and supportive measures alone.
INTRODUCTION
Acute renal failure (ARF) has become a rare complication with pregnancy in industrialized countries. In the developing world, pregnancy-related ARF continues to account for 20% of total ARF cases, and mortality rates remain as high as 50%.Citation[1]
Postpartum renal failure is associated most often with preeclampsia and/or hypertension; Hemolysis, Elevated Liver Enzymes, Low Platelets (HELLP) Syndrome; hemolytic uremic syndrome; or thrombotic thrombocytopenic purpura. We are presenting a case of ARF in early postpartum which on investigations turned out to be a case of bilateral emphysematous pyelonephritis.
CASE REPORT
A 29-year-old female who had full-term, normal, vaginal delivery four days previously presented to us with severe, non-colicky pain in the left flank along with nausea, vomiting, abdominal distension, and oligoria two days in duration. She had no history of fever, trauma, dysuria, frank hematuria, or excessive discharge/bleeding per vaginam. Her previous two pregnancies were uneventful, and she did not have any complication during the current pregnancy. She had no history of diabetes, hypertension, tuberculosis, or renal disease. Her physical examination at presentation in hospital revealed cool clammy extremities, tachycardia (pulse rate of 120 per/min), hypotension (systolic blood pressure of 60 mm Hg), pallor, and bilateral pitting pedal edema. Her respiratory and cardiac examination was unremarkable, but abdominal examination revealed a distended, nontender abdomen without organomegaly or free fluid and bilateral renal angle tenderness. Her per-rectal examination was normal and per-vaginam examination showed closed os and well-contracted uterus with no forniceal tenderness.
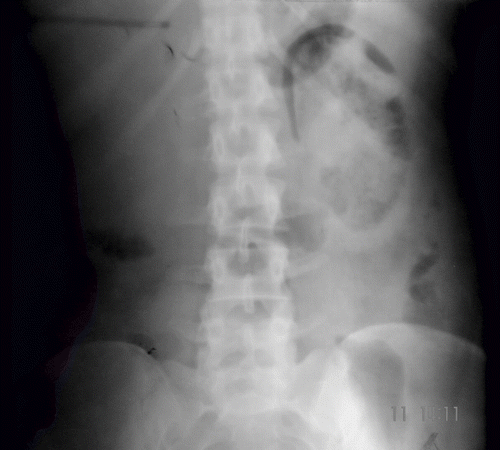
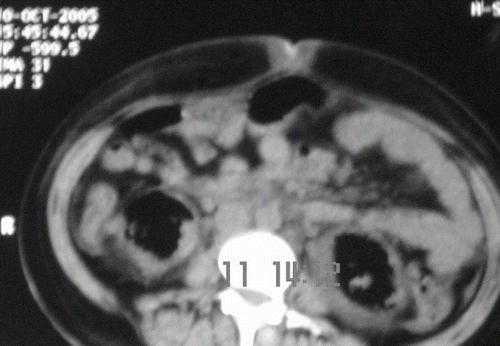
Blood investigation showed anemia (Hb 7gm/dL), polymorphonuclear leucocytosis (TLC 16,800/dL with 86% neutrophils), and thrombocytopenia (count 30,000/dL) without any evidence of hemolysis. Random blood sugar was 80 mg/dL, blood urea was 142 mg/dL (50.69 mmol/L), serum creatinine of 3 mg/dL (265.2 μmol/L), and serum uric acid was 4.2 mg/dL. Liver function test was essentially normal. Blood coagulogram was within normal limits, and ANA was negative. Urinalysis showed 50–100/HPF pus cells, 30–40/HPF fresh RBCs, and albuminuria. Urine culture showed significant growth of Escherichia coli and Klebsiella. Arterial blood gas analysis showed high anion-gap metabolic acidosis. Ultrasound examination at presentation showed normal-sized kidneys with right-sided renal cortical infarcts; however, follow-up ultrasound two days later showed bilateral renal parenchymal and perinephric air echoes, more marked on the left side. Doppler ultrasound of renal artery and vein was normal. A plain X ray KUB (see ) revealed gas shadows streaking the renal parenchyma as well as outlining the kidneys bilaterally. A noncontrast CT (see ) of abdomen revealed bilaterally enlarged kidneys with evidence of intraparenchymal air with extension into the perinephric fascia, suggestive of bilateral emphysematous pyelonephritis.
Figure 2. Non-contrast computer tomographic scan of abdomen showing air echos in bilateral renal parenchymal and perinephric space, more marked on the left side.
A diagnosis of Type 1 Class 4 emphysematous pyelonephritis was made, and in addition to inotropic and supportive treatment, she was started on parenteral antibiotics (ceftriaxone 1 gm i.v. twice daily and metronidazole 500 mg thrice daily) and initiated on hemodialysis because of oliguria and deranged renal function. Surgical drainage/nephrectomy was denied in view of high risk on account of thrombocytopenia, shock, deranged renal function, and bilateral renal involvement. After the receipt of urine culture, sensitivity pattern antibiotics were changed to modified dose of levofloxacin. After four dialysis sessions, her general condition improved, and by the tenth day of admission, her urine output had increased to 1 liter per day. It took five weeks for her renal parameters to improve. At discharge, her serum creatinine was 1.8 mg/dL (160.12 μmol/L), indicating some degree of residual renal damage. Investigation for risk factors for EPN could not identify any known risk factor.
DISCUSSION
Peripartum acute renal failure is an important clinical problem, and despite decreasing incidence, it is associated with significant mortality and morbidity. In general, causes could be grouped under several categories of pregnancy-specific ARF: hypovolemic, thrombotic microangiopathic, infectious, and obstructive, or there could be worsening of pre-existing renal failure. Our case presented with pain abdomen, shock, and oliguric renal failure in the early postpartum period. She neither had history of pre-existing renal disease, and neither the first ultrasound nor the CT scan gave any indication of pre-existing chronic renal disease or obstructive etiology. She did not have major blood loss in the peripartum period; in addition, the possibility of renal hypoperfusion due to a renal vascular occlusion was excluded by Doppler ultrasound.
Preeclampsia typically develops in the late third trimester, including the intrapartum period; only a few percent of cases develop in the postpartum period, and then usually in the first 24 to 48 hours. Renal failure is relatively unusual even with severe cases, unless there is significant bleeding with hemodynamic instability or marked disseminated intravascular coagulation (DIC). Frank renal failure and absence of increased blood pressure in our patient excluded the possibility of preeclampsia. Although our patient had thrombocytopenia and anemia, in view of absence of microangiopathic hemolytic picture, the possibility of thrombotic thrombocytopenic purpura-hemolytic uremic syndrome (TTP-HUS) was ruled out. Normal liver function tests and absence of hemolysis ruled out acute fatty liver of pregnancy and HELLP syndrome.
Bilateral renal cortical necrosis may be induced during pregnancy by abruptio placentae or other severe complications such as placenta previa, prolonged intrauterine fetal death, or amniotic fluid embolism.Citation[2] Affected patients typically have one of the above complications of pregnancy and then develop abrupt onset of oliguria or anuria, frequently accompanied by gross hematuria, flank pain, and hypotension. The triad of anuria, gross hematuria, and flank pain is unusual in other causes of renal failure in pregnancy. In our patient, there was no complication during the pregnancy, and pain was there in left flank only. The USG abdomen showed some cortical infarcts only in the right kidney.
Pneumaturia secondary to renal infection by gas-forming uropathogens was first described by Kelly and MacCullum in 1898. Emphysematous pyelonephritis has increasingly been described in patients with poorly controlled diabetes, which accounts for 70–90% of all reported cases.Citation[3],Citation[4] In rare cases occurring in non-diabetics, urinary tract obstruction, polycystic kidney disease, and end stage renal disease were found to be the contributing factors. The factors that have been identified to predispose a patient to its development are old age, female sex, diabetes, elevated glycosylated hemoglobin (>8%), nephrolithiasis, urinary tract obstruction, and immunodeficiency. The left kidney is affected more commonly than the right, with bilateral cases constituting less than 10% of cases.Citation[5] Bilateral emphysematous pyelonephritis is a life-threatening condition usually occurring in diabetics or those with urinary tract obstruction. Few cases have been documented in literature, which have occurred in absence of these predisposing factors.Citation[6],Citation[7] The organisms most commonly associated with EPN are E. coli, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa, Aerobacter aerogenes, Citrobacter, and rarely yeast. Mixed infections are found in 10% of cases of EPN.
It has been postulated that the development of EPN requires the presence of gas-forming organism, high tissue glucose, impaired tissue perfusion, and gas transport and defective immunity.Citation[8–10] It had been suggested that poor glycemic control rather than the diabetes itself is the predisposing factor for development of EPN.Citation[11] In nondiabetics, the presence of an impaired vascular supply manifested by intrarenal thrombi or renal infarction is the cause. In the present case, impaired vascular supply as evidenced by renal cortical infarcts on ultrasonography may be the reason for development of EPN, as there was no evidence of diabetes or urinary tract obstruction. Pregnancy is not considered a risk factor for EPN, although urinary tract infections are the most common bacterial infection occurring during pregnancy, and pyelonephritis is the most common severe bacterial infection complicating pregnancy.Citation[12] Hormonal changes associated with pregnancy lead to the increased incidence of pyelonephritis, the greatest being at the end of the second and beginning of the third trimester. Peripartum state is a predisposing factor for asymptomatic bacteruria, urinary tract infection, and pyelonephritis, but has not been considered as a predisposing factor for emphysematous pyelonephritis so far. In the presence of impaired blood supply, these asymptomatic urinary infections may lead to development of EPN, as there is some degree of vascular insufficiency that impairs the transport of gases produced as a result of tissue catabolism, leading to their accumulation within tissues.
Bilateral EPN is very rare and carries very high mortality.Citation[5] A recent meta-analysis of 175 patients with emphysematous pyelonephritis has shown that the main factors associated with increased mortality include conservative treatment alone, bilateral emphysematous pyelonephritis, type 1 emphysematous pyelonephritis, and thrombocytopenia. Despite the aggressive treatment, these patients have overall a 25% mortality rate. This meta-analysis did not show any association between diabetes and mortality in patients with emphysematous pyelonephritis, and so was the case with nephrolithiasis and infection due to E. coli or Klebsiella. However, increased serum creatinine level, disturbance of consciousness, and hypotension (systolic blood pressure < 90 mmHg) may also impact the mortality rate of these patients, though there is limited data supporting these factors.Citation[13]
Treatment modalities usually depend on the class of EPN at the time of presentation and the presence or absence of the following risk factors: shock, thrombocytopenia, altered sensorium, and impaired renal function. Because of the rarity of this disorder, definite guidelines for optimal management are yet to be established. Nephrectomy is generally regarded as the standard of care for the management of emphysematous pyelonephritis in patients capable of undergoing surgery.Citation[14] However, this is itself a hazardous intervention in a septic, unstable patient with circulatory or liver failure. When bilateral disease is present, the need for long-term dialysis is obviously unavoidable. Conservative management or percutaneous drainage has occasionally been advocated, primarily for patients for whom surgery is not a suitable option. Unfortunately, the rate of treatment failure associated with these conservative management strategies is usually prohibitively high.Citation[15],Citation[16] Several individual case reports of EPN treated successfully with antibiotics alone have been described in literature.Citation[4] Overall, still there remains controversy about optimal treatment of emphysematous pyelonephritis. However, with the advent of CT scanning, more powerful antibiotics, and better access to life support, an alternative medical approach to radical surgery has emerged.Citation[17],Citation[18]
In the present case, there is involvement of bilateral kidney, so to preserve renal function, conservative treatment was a better option rather than going for bilateral nephrectomy. Nephrectomy generally should not be performed in patients with bilateral emphysematous pyelonephritis or with emphysematous pyelonephritis of solitary kidney, in view of preserving renal function. It is advocated that nephrectomy should be performed if the contralateral kidney is not affected.
This case is unique as it represents the first reported case of bilateral emphysematous pyelonephritis in a post partum nondiabetic female without evidence of urinary tract obstruction. Further, mixed bacterial infection with gas producing organisms and successful conservative management of Class 4, Type 1 EPN by antibiotics and hemodialysis alone are even rarer occurrence.
Impaired renal blood supply can itself predispose to EPN without other predisposing factors, and whether peripartum state is a predisposing factor for EPN needs to be further validated. To provide a consensus guideline for the management of this rare, life-threatening condition is still a challenge.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Gammill H, Jeyabalan A. Acute renal failure in pregnancy. Crit Care Med. 2005; 33(Suppl)S372–S384
- Matlin RA, Gary NE. Acute cortical necrosis. Case report and review of the literature. Am J Med. 1974; 56: 110–118
- Schultz EH, Klorfein EH. Emphysematous pyelonephritis. J Urol. 1962; 87: 762–766
- Flores G, Nellen H, Magaña F, Calleja J. Acute bilateral emphysematous pyelonephritis successfully managed by medical therapy alone: A case report and review of the literature. BMC Nephrology. 2002; 3: 4
- Stein JP, Spitz A, Elmajian DA, et al. Bilateral emphysematous pyelonephritis: A case report and review of the literature. Urology. 1996; 47: 129–134
- Pandey S, Kumar S, Dorairajan LN, et al. Emphysematous perinephric abscess without diabetes or urinary obstruction. Urol Int. 2003; 71: 322–324
- Hart PD, Vaseemuddin M, Egiebor O, Dunea G. Bilateral emphysematous pyelonephrotis in a patient with no known risk factors. JAMA. 2007; 99(2)179–181
- Huang JJ, Tseng CC. Emphysematous pyelonephritis: Clinico-radiological classification, management, prognosis and pathogenesis. Arch Intern Med. 2000; 160: 791–805
- Ireland GW, Javadpore N, Cass AS. Renal emphysema and retention of renal function. J Urol. 1971; 106: 463–466
- Huang JJ, Chen KW, Ruaan MK. Mixed acid fermentation of glucose as a mechanism of emphysematous urinary tract infection. J Urol. 1991; 146: 148–151
- Tseng CC, Wu JJ, Wang MC, et al. Host and bacterial virulence factor predisposing to emphysematous pyelonephritis. Am J Kidney Dis. 2005; 46: 432–439
- Cunningham FG, Lucas MJ. Urinary tract infections complicating pregnancy. Baillieres Clin Obstet Gynaecol. 1994; 8: 353–373
- Falagas ME, Alexiou VG, Giannopoulou KP, Siempos II. Risk factors for mortality in patients with emphysematous pyelonephritis: A meta-analysis. J Urol. 2007; 178(3)880–885, Shokeir AA, El-Azab M, Mohsen T, et al. Emphysematous pyelonephritis: A 15-year experience with 20 cases. Urology. 1997;49:343–346
- Shokeir AA, El-Azab M, Mohsen T, et al. Emphysematous pyelonephritis: A 15-year experience with 20 cases. Urology 1997, 49: 343–346
- Klein FA, Smith MJ, Vick CW, III, et al. Emphysematous pyelonephritis: diagnosis and treatment. South Med J. 1986; 79: 41–46
- Ahlering TE, Boyd SD, Hamilton CL, et al. Emphysematous pyelonephritis: A five-year experience with 13 patients. J Urol. 1985; 134: 1086–1088
- Grozel F, Berthezene Y, Guerin C, et al. Bilateral emphysematous pyelonephritis resolving to medical therapy: Demonstration by US and CT. Eur Radiol. 1997; 7(6)844–846
- Guerin C, Noel P, de Varax R, et al. Bilateral emphysematous pyelonephritis cured by medical therapy alone. Intensive Care Med. 1997; 23(8)921–922