Abstract
Background/aim. Post-transplant cardiovascular events are associated with increased morbidity and mortality after renal transplantation. Though renal transplantation eliminates cardiovascular disease risk factors by restoring renal function, it introduces new cardiovascular risks derived partly from immunosuppressive medications. In this study, to assess the effects of various immunosuppressive drugs on platelet function of renal transplant patients, we measured soluble P selectin levels (sP-selectin) and performed platelet aggregation studies in patients who have undergone renal transplantation. Methods. sP-selectin levels and platelet aggregation induced by 5 μM adenosine diphosphate (ADP), 5 μM epinephrine, 1.25 mg/mL ristocetin, and 2 μg/mL collagen were studied by whole blood platelet lumi-aggregometer in 40 renal transplant patients. Patients in group 1 (n = 24) were treated with cyclosporine/mycophenolate mofetil/methylprednisolone, and group 2 (n = 16) were treated with tacrolimus/mycophenolate mofetil/methylprednisolone. Effects were compared with those in control groups of hypertensive subjects and healthy subjects. Results. Platelet aggregation values induced by ADP, epinephrine, ristocetin, and collagen were lower in cyclosporine-treated patients than tacrolimus-treated patients, hypertensive subjects, and healthy subjects, though the difference was not statistically significant (p > 0.05). sP-selectin levels were appreciably higher in cyclosporine-treated patients, and statistically significant differences were observed compared with those of tacrolimus-treated patients (p < 0.05), hypertensive subjects (p < 0.01), and healthy subjects (p < 0.05). Conclusion. We conclude that cyclosporine-treated renal transplant patients show enhanced platelet activation in which anti-platelet therapy should be considered, in addition to management of other conventional cardiovascular risk factors, to decrease the cardiovascular morbidity and mortality in this high risk population.
INTRODUCTION
Renal transplantation is currently the best mode of therapy for virtually all suitable candidates with end-stage renal disease. Compared with dialysis, successful kidney transplantation improves the quality of life and reduces the mortality risk for most patients.Citation[1] However, post-transplant cardiac complications increase the morbidity and mortality rates after renal transplantation.Citation[2] Cardiovascular disease is the leading cause of death in renal transplant patients accounting for 40–55% of all deaths, and the incidence is considerably higher than in the general population.Citation[3] The main contributing factors leading to atheroma include older age, male gender, hyperlipidemia, smoking, obesity, lack of exercise, hyperglycemia, and hyperhomocysteinanemia.Citation[4]
Though renal transplantation eliminates cardiovascular disease risk factors by restoring renal function, it introduces new cardiovascular risks, derived in part from immunosuppressive medications.Citation[1] Immunosuppressive therapy necessary to avoid premature graft loss may also increase the tendency to atheroma. Hypercoagulability is thought to contribute to the development and progression of atherosclerosisCitation[2],Citation[5] and is generally attributed to combined abnormalities in platelet and coagulation-dependent hemostasis in transplanted patients.Citation[6],Citation[7] Changes in platelet functionCitation[8],Citation[9] and fundamental classes of coagulation components, including alterations in clotting factors, fibrinogen, and clotting inhibitors,Citation[10] as well as fibrinolytic system abnormalitiesCitation[11] may play a role in the prothrombotic state observed in renal transplant patients treated with cyclosporine.
In the present study, to assess the effects of various immunosuppressive drugs on platelet function of renal transplant patients, we measured levels of soluble p selectin (sP-selectin), a marker of platelet activation, and performed platelet aggregation studies.
PATIENTS AND METHODS
Patients
A total of forty renal transplant patients were included in the study. The patients were divided into two groups according to their immunosuppressive regimens. Patients in group 1 (n = 24; age, mean 32.20 ± 9.01 years, 15 males and 9 females) were treated with cyclosporine/mycophenolate mofetil/methylprednisolone, and those in group 2 (n = 16; age, mean 36.37 ± 10.66 years, 9 males and 7 females) were treated with tacrolimus/mycophenolate mofetil/methylprednisolone. All of them maintained stable renal function (serum creatinine level < 2.5 mg/dL) and showed no signs of rejection.
Exclusion criteria included patients with known bleeding or other systemic disorders such as hepatic and endocrine diseases, acute infections, autoimmune disorders, or cancer; a platelet count less than 150 × 109/L or more than 450 × 109/L and a hemoglobin level less than 10 g/dL. The patients did not receive aspirin, ticlopidine, dipyridamole, or nonsteroidal antiinflammatory drugs in the ten days prior to the platelet aggregation studies while allowed the concomitant use of antihypertensive drugs. None of the patients received EPO, IV iron, or active vitamin D.
Fifteen patients with hypertension who were taking efficient antihypertensive medications similar to that given to the renal transplant patients (group 3; age, mean 47.0 ± 13.63 years, 5 males and 10 females) and 13 healthy subjects served as control groups (group 4; age, mean 28.30 ± 3.44 years, 13 males). Body mass index (BMI) was calculated as weight (kg) divided by the square of height (m). The glomerular filtration rate (GFR) was assessed according to the formula of Cockcroft and Gault.
All of the participants gave written informed consent, and the Ethical Committee of Eskisehir Osmangazi University Medical School (Eskisehir, Turkey) approved the study.
Immunosuppressive Therapy
Immunosuppression regimen included cyclosporin A or tacrolimus and mycophenolate mophetil (2 × 1 g) or mycophenolic acids (2 × 720 mg) and maintenance steroid. Cyclosporin A and tacrolimus concentrations were controlled according to standard protocols. Mycophenolate mophetil and mycophenolic acid dosage was adjusted in case of intolerance.
Sample Collection and Laboratory Methods
Venous blood was drawn into 4.5 ml vacutainers (Becton Dickinson) containing 3.8% trisodium citrate in a 9:1 blood anticoagulation ratio by direct vein puncture with 19 gauge needles early in the morning after overnight fasting.
The platelet aggregation specimens were kept at room temperature and processed within one hour of blood collection. The remaining blood samples were immediately immersed in an ice bath and centrifuged at 2500 g at 4°C for 20 min, and plasma was stored in aliquots at −70°C until it was used.
Sample for blood counts were drawn into Becton Dickinson anticoagulated tubes, and complete counts were made by a Beckman Coulter Gen-S SM automated blood counting device.
Platelet aggregation studies were performed in whole blood lumi-aggregometer (Chronolog Corporation, Model 540-Ca) using an optical method according to manufacturer's instructions. Whole blood specimens were centrifuged for 10 min at 200 g to obtain platelet-rich plasma. Platelet poor plasma was obtained on the remaining specimen by re-centrifugation at 200 g for 15 min. A platelet count was performed on the platelet-rich plasma and was adjusted to 300 × 103/μL with platelet poor plasma. 450 μL of this platelet rich plasma were transferred into cuvettes (Chronolog No: P/N 312) each containing a disposable siliconized bar. After agonist addition, platelet aggregation was measured over 6 min and expressed as a percentage of the maximal amplitude in PRP. The agonist used and their final concentrations were ADP (Chrono Par 384), 5 μM; collagen (Chrono Par 385), 2 μg/mL; ristocetin (Chrono Par 396), 1.25 mg/mL; and epinephrine (Chrono Par 393), 5 μM.
Coagulation tests were performed on ACL TOP Coagulation Analyzer (Instrumentation Laboratories). Prothrombin time (PT) was measured by HemosIL RecombiPlasTin kit (Instrumentation Laboratory), activated partial thromboplastin time (APTT) was measured by HemosIL SynthASil kit (Instrumentation Laboratory), fibrinogen was measured by HemosIL Fibrinogen-C XL kit (Instrumentation Laboratory), and D-Dimer was measured by HemosIL D-Dimer kit (Instrumentation Laboratory). The normal ranges for these tests in our laboratory are APTT, 24–36 s; PT, 8–13 s; fibrinogen, 200–400 mg/dL; and D-Dimer, 0–500 μg/dL. Commercially available ELISA method was used to determine sP-selectin (Bender MedSystems, Vienna, Austria; normal value 111–266 ng/mL). All analyses were performed in duplicate, and the mean value was used for statistically calculations.
Statistical Analysis
SPSS for Windows 16.0 (SPSS Inc, Chicago, Illinois, USA) and Sigmastat 3.1 were used in analyzing the data. The distribution of variables was checked initially by Shapiro Wilks test. Parametric tests were applied to data having normal distribution, whereas nonparametric tests were applied to data having non-normal distribution. Chi-square tests were applied for categorical variables. One-way ANOVA test and Kruskal-Wallis One-Way Analysis of Variance on Ranks Test were applied to determine the difference between independent four groups. In addition, Tukey HSD and Dunn's Post Hoc Tests were applied for checking the differences. The relationships among the variables were evaluated by using Pearson and Spearman's rho correlation analysis.
Results were expressed as mean ± SD and median (interquartile range), and p value <0.05 was considered statistically significant.
RESULTS
Study Subjects
Baseline characteristics of patient and control groups are summarized in . Among renal transplant patients, no significant differences in terms of age, body mass index, glomerular filtration rate, and methylprednisolone dose were noticed. Months since transplantation was significantly higher in cyclosporine-treated patients, while the duration of pre-transplantation dialysis (months) was significantly higher in tacrolimus-treated patients. Renal transplant patients and hypertensive controls trended to have similar antihypertensive agents and had adequate blood pressure control.
Table 1 Baseline characteristics of the study population
Hematologic and Biochemical Assays
Hemoglobin and platelet values were similar in cyclosporine- and tacrolimus-treated patients. Plasma levels of APTT were found to be significantly decreased in cyclosporine- and tacrolimus-treated patients compared with healthy controls (p < 0.001), whereas PT levels showed no significant difference between the patient and control groups. Plasma fibrinogen levels were considerably higher in patients taking cyclosporine compared with hypertensive (p < 0.01) and healthy controls (p < 0.05). Serum D-Dimer levels were higher in both groups of renal transplant patients than those of healthy controls (p < 0.05; see ). No significant difference was observed in fasting glucose, calcium, and phosphorus levels between all groups of patients and control subjects. Serum triglyceride levels were significantly higher in cyclosporine- and tacrolimus-treated patients than healthy controls (p < 0.05), while serum HDL cholesterol levels were found to be higher in tacrolimus-treated patients than cyclosporine-treated patients (p < 0.05), hypertensive controls (p < 0.05), and healthy controls (p < 0.001). Serum LDL cholesterol levels were higher in cyclosporine-treated patients than tacrolimus-treated patients and hypertensive and healthy controls, although the difference was not statistically significant (p > 0.05; see ).
Table 2 Laboratory parameters of the study population
Platelet Aggregation and sP-Selectin Assays
Platelet aggregation values induced by 5 μM ADP, 5 mM epinephrine, 1.25 mg/mL ristocetin, and 2 μg/mL collagen are shown in . As can be seen from the table, platelet aggregation values induced by all agonists were lower in cyclosporine-treated patients than tacrolimus-treated patients and hypertensive and healthy controls, but the difference did not reach statistical significance (p > 0.05; see ). There was a negative correlation between cyclosporine level and platelet aggregation values induced by ADP (r = 0.42, p < 0.05), ristocetin (r = 0.42, p < 0.05), and collagen (r = 0.53, p < 0.01), while there was no correlation between tacrolimus level and platelet aggregation values induced by all agonists.
Table 3 Platelet aggregation studies and sP-selectin levels
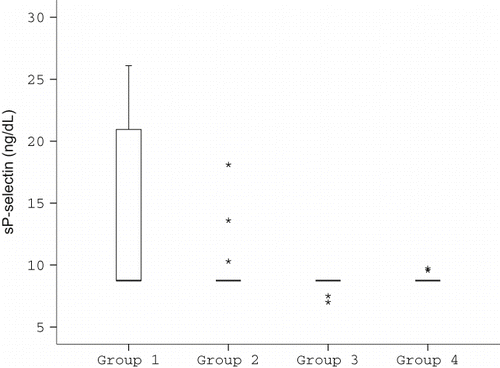
sP-selectin levels were appreciably higher in cyclosporine-treated patients, and statistically significant differences were observed compared with those of tacrolimus-treated patients (p < 0.05), hypertensive subjects (p < 0.01), and healthy subjects (p < 0.05; see and ). Cyclosporine and tacrolimus levels did not show any correlation with sP-selectin levels.
DISCUSSION
New immunosuppressive agents reduced the number of cardiovascular events in renal transplant patients. However, post-transplant cardiovascular mortality is still an important problem. In addition to conventional coronary risk factors, coagulation abnormalities play a key role in the hypercoagulable state observed in transplanted patients. As a matter of fact, there is no consensus about the evaluation and the prevention of post-transplant coagulation abnormalities. Therefore, to clarify this issue, we aimed to investigate the effects of immunosuppressive drugs on platelet function of renal transplant patients by soluble P-selectin measurement as a marker of platelet activation and platelet aggregation testing in the present study.
Atherosclerotic diseases account for a high morbidity and mortality in renal transplant patients.Citation[2] Immunosuppressive therapy, which is necessary to avoid premature graft loss, increases the tendency to atheroma. Tacrolimus, cyclosporine (to a greater degree than tacrolimus), and corticosteroids have been implicated in post-transplant hypertension. Corticosteroids, tacrolimus, and to a lesser extent, cyclosporine have been shown to be associated with an increased risk for new-onset diabetes mellitus after transplantation.Citation[12] The hyperlipidemic effect of immunosuppressive medication, including corticosteroids, cyclosporine, tacrolimus, and sirolimus, has been also well-documented. Additionally, in the post-transplant setting, excessive weight gain or obesity may become a problem for many patients on steroid therapy.Citation[1] Studies including liver transplant recipients revealed that cyclosporine immunosuppression is associated with a higher likelihood of post-transplant weight gain compared with tacrolimus.Citation[13] Our data reporting an increase of serum triglyceride and LDL cholesterol levels and a decrease of serum HDL levels in cyclosporine-treated patients support post-transplant dyslipidemia. We agree that conventional coronary risk factors in the post-transplant setting should be identified and managed aggressively to decrease the cardiovascular morbidity and mortality in this high-risk population.
Disturbances in platelet function have been proposed to account for the increased risk of the thrombotic complications associated with immunosuppressive drugs. Enhanced in vivo and in vitro platelet aggregation responses with cyclosporine have been reported and associated with the thrombotic tendency in those patients.Citation[6],Citation[14],Citation[15] Data on thromboembolic events under treatment with tacrolimus is controversial, while the effect of mycophenolate mophetil on post-transplant thrombotic complications has not been described.Citation[16] In an in vitro study by Malyszko et al.,Citation[8] preincubation of human platelets with tacrolimus and mycophenolate mophetil resulted in a significant decrease in platelet aggregation responses,Citation[16] whereas tacrolimus-induced enhancement of platelet aggregation has been shown by Klein et al.Citation[16] In the present study, we did not observe significant differences between cyclosporin- and tacrolimus-treated patients in terms of platelet aggregation values. Platelet aggregation values were found to be negatively correlated with cyclosporine concentrations, but did not show any significant correlation with tacrolimus concentrations.
Activated platelets and endothelial cells rid themselves of sP-selectin, and these activated platelets have been described as a direct inducer in procoagulant activity in addition to being a marker of inflammation.Citation[17],Citation[18] Raised levels of sP-selectin predict adverse thromboembolic events such as stroke and myocardial infarction.Citation[19–21] Data on platelet activation among renal transplant patients are limited to a single study by Graff et al.,Citation[9] in which all renal transplant patients had enhanced platelet activation, as indicated by expression of CD62, a marker of platelet degranulation. Our results reporting markedly raised levels of soluble P-selectin in cyclosporine-treated renal transplant patients compared with those of the patients taking tacrolimus are in good agreement with the above findings.
In conclusion, the results of this study suggested that cyclosporine-treated patients seemed to exhibit enhanced platelet activation in which anti-platelet therapy, aspirin, or other anti-platelet agents should be promptly recommended. Considering high rates of post-transplant cardiac mortality, we believe that accurate diagnosis and aggressive management of conventional cardiovascular risk factors like effective blood pressure control, intensive glycemic control, treatment of dyslipidemia, and smoking cessation are important in patients who had undergone renal transplantation.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Pham PT, Pham PC, Danovitch GM. Cardiovascular disease posttransplant. Semin Nephrol. 2007; 27(4)430–444
- Sato K, Ogawa K, Onumata O, et al. Cause of death in renal transplant patients: A comparison between azathioprine and ciclosporin. Surg Today. 2001; 31: 681–687
- Aker S, Ivens K, Grabensee B, Heering P. Cardiovascular risk factors and diseases after renal transplantation. Int Urol Nephrol. 1998; 30(4)777–788
- Briggs JD. Causes of death after renal transplantation. Nephrol Dial Transplant. 2001; 16: 1545–1549
- Ross R. The pathogenesis of atherosclerosis: An update. N Engl J Med. 1986; 314: 488–500
- Vanrenterghem Y, Roels L, Lerut T, et al. Tromboembolic complications and haemostatic changes in cyclosporine-treated cadaveric kidney allograft recipients. Lancet. 1985; 1: 999–1002
- Grace AA, Barradas MA, Mikhailidis DP, Jeremy JY, Sweny P, Dandona P. Cyclosporine A enhaces platelet aggregation. Kidney Int. 1987; 32(6)889–895
- Malyszko J, Malyszko JS, Takada A, Mysliwiec M. Effects of immunosuppressive drugs platelet aggregation in vivo. Ann Transplant. 2002; 7(1)55–68
- Graff J, Klinkhardt U, Harder S, et al. Immunosuppressive therapy regimen and platelet activation in renal transplant patients. Clin Pharmacol Ther. 2002; 72(4)411–418
- Malyszko J, Malyszko JS, Pawlak K, Michal M. The coagulo-lytic system and endothelial function in cyclosporine-treated kidney allograft recipients. Transplantation. 1996; 62(2)828–830
- Verpooten GA, Cools FJ, Van der Planken MG, et al. Elevated plasminogen activator inhibitor levels in cyclosporine-treated renal allograft recipients. Nephrol Dial Transplant. 1996; 11: 347–351
- Opelz G, Wujciak T, Ritz E, et al. Association of chronic kidney graft failure with recipient blood pressure. Kidney Int. 1998; 53: 217–222
- Canzanello VJ, Schwartz L, Tater SJ, et al. Evolution of cardiovascular risk after liver transplantation: A comparison of cyclosporine A and tacrolimus FK(506). Liver Transpl Surg. 1997; 3: 1–9
- Grace AA, Barradas MA, Mikhailidis DP, et al. Cyclosporine A enhances platelet aggregation. Kidney Int. 1987; 32(6)889–895
- Fernandes JB, Naik UP, Markell MS, Kornecki E. Comparative investigation of the effects of the immunusuppresants cyclosporine A, cyclosporine G, and FK-506 on platelet aggregation. Cell Mol Biol Res. 1993; 39(3)265–274
- Klein BC, Bach D, Rehfeld I, et al. Influence of mycophenolic acid and FK-506 on human platelet activation in vitro. Kidney Blood Press Res. 2000; 23(2)119–124
- Blann AD, Lip GY. Hypothesis: Is soluble P-selectin a new marker of platelet activation?. Atherosclerosis. 1997; 128: 135–138
- Andre P, Hartwell D, Hrachovinova I, Saffaripour S, Wagner DD. Pro-coagulant state resulting from high levels of P-selectin in blood. Proc Natl Acad Sci USA. 2000; 97: 13835–13840
- Blann AD, Fargher EB, McCollum CN. Increased soluble P-selecyn in ischaemic heart disease: A new marker for the progression of atherosclerosis. Blood Coagul Fibrinolys. 1997; 8: 383–390
- Ridker PM, Buring JE, Rifai N. Soluble P-selectin and the risk of future cardiovascular events. Circulation. 2001; 103: 491–495
- Hillis GS, Terregino C, Taggart P, Killian A, Zhao N, Dalsey WC. Elevated soluble P-selectin levels are associated with an increased risk of early adverse in patients with presumed myocardial infarction. Am Heart J. 2002; 143: 235–241