Abstract
Background. Nephropathy associated with contrast medium exposure is a well-known complication of IVP. However, it is uncertain whether iso-osmolar non-iodinated contrast medium (iodixanol) is less nephrotoxic than low-osmolar contrast medium (iohexol). Materials and Methods. In this single-center, double-blind, prospective study, 50 patients undergoing IVP were randomized into two groups receiving different contrast medium: iodixanol and iohexol. Patients in high risk for contrast nephropathy were included, 28 with renal insufficiency and 19 with diabetes mellitus. We compared the nephrotoxic effect (contrast nephropathy), complement and cytokines profile between the iodixanol and iohexol groups. The mean volume of contrast medium in each IVP procedure was 0.8 mL/kg. Results. The incidence of contrast nephropathy was 4 percent among all patients (one iodixanol and one iohexol). We found no significant differences in contrast nephropathy and allergic reactions between the two groups. There was no significant difference in cytokine profiles in both groups (p > 0.05).The incidence of allergic reaction was 16 percent among all patients. Twelve percent (3/25) had late reaction after iohexol exposure compared to four percent (2/25) with iodixanol (p = 1.0). One patient had severe skin rash due to late adverse reaction after iodixanol. No mortality was found. Conclusions. New iodixanol and iohexol contrast medium for routine IVP examination are safe and have low nephrotoxicity profile, especially in elderly or high-risk patients. Iodixanol contrast medium has an increased risk to induce severe late adverse reaction compared to iohexol. Allergic reaction may be the main adverse effect after contrast medium infusion.
INTRODUCTION
Contrast nephropathy (CN) is a complication of arteriographic procedures, but also of intravenous pyelography (IVP) and computed tomography scans with use of intravenous contrast media.Citation[1–5] Routine IVP was performed in nephrology and urology.Citation[3],Citation[4] However, the use of iodinated contrast medium (CM) resulting in CN may not be entirely avoidable. In spite of the improvements in the chemical structure of contrast media, CN is still the third leading cause of hospital-acquired acute renal failure.Citation[5]
Both pre-existing renal insufficiency and diabetes mellitus have been demonstrated to significantly increase the risk of CN (so-called high-risk patients).Citation[6],Citation[7] During the past decade, nonionic (low-osmolality) CM have become increasingly popular for radiographic procedures requiring intravascular contrast because they are associated with a decreased incidence of systemic and organ toxicity compared to conventional ionic (high-osmolarity, iothalamate meglumine injection USP 60%) contrast media. Moreover, data from animal studies have suggested that nonionic CM is less nephrotoxic than ionic CM.Citation[8–12]
CN can be defined as an acute impairment of renal function following the exposure of radiocontrast medium.Citation[1] The clinical presentation of CN is distinct, having a temporal relation between the performance of the contrast study in the high-risk patient and the onset of an increase in serum creatinine levels within the next 24 hours to 5 days. Serum creatinine values greater than 25% of baselineCitation[6] or rising by 0.5 mg/dL or more are diagnostic.Citation[10] The peak serum creatinine level occurs within 3–5 days of the contrast enhanced study. Monitoring serum creatinine is the most useful clinical approach in high-risk patients after IVP.
Several studies have provided evidence that CM have direct cytotoxic effects on the renal structures. Until now, several pathophysiological mechanisms have been proposed, including disturbed renal perfusion/hypoxia, direct toxicity to renal tubular epithelium, apoptosis, altered glomerular function, and immunologic mechanisms.Citation[3–22] Histamine is a well-known mediator of inflammation and a very important modulator of cytokine production.Citation[23] Previous studies have reported on the elevation of plasma histamine levels after conventional CM.Citation[24],Citation[25] However, no large serial report has examined plasma histamine release after iodixanol or iohexol after IVP in Taiwan. Thus, complement and cytokines profiles are to be measured for this purpose. Prophylactic measures include hydration, diuresis, mannitol and renal vasodilators, etc.Citation[5–27] There is no evidence that the use of mannitol, furosemide, aminophylline, natriuretic peptide, or low-dose dopamine provides any significant prophylactic benefit. The use of aggressive hydration strategies before contrast infusion has been shown to be important in preventing CN. Severe complications in the high-risk patients, including adverse reactions of CM,Citation[29–31] may be managed by hemodialysis.Citation[28]
In the past, non-ionic monomers and dimmers have both been shown to have a good safety profile in normal subjects. Patients with renal impairment are at increased risk of developing CN. There are limited clinical data in the nephrotoxicity of iodixanol in renal impairment. However, previous reportsCitation[32],Citation[33] with regard to CN after IVP in high-risk patients are primarily from western countries, and very few are from Taiwan. We designed a prospective, controlled study to compare the incidence of contrast nephrotoxicity between the iodixanol and iohexol contrast agent, especially in elderly or high-risk patients undergoing IVP. Serum creatinine and immunologic reaction are to be measured pre- and post-IVP in a series of in-patients to establish whether there is any clinically detectable evidence of superiority of iodixanol over iohexol CM.
MATERIALS AND METHODS
A total of 50 patients receiving IVP in our hospital from September 1, 2005, to August 31, 2006, were randomized to receive iodixanol or iohexol in this study. Exclusion criteria were pregnancy, volume depletion or fluid overload, IV-iodinated CM within seven days, treatment with metformin or NSAID within 48 hrs, and nephrotoxic drugs within seven days. All study subjects provided written informed consent, and the ethical committee approved this protocol. This study included diabetes patients with serum creatinine less than 1.5 mg per deciliter, diabetic patients with preexisting renal insufficiency (defined by serum creatinine more than 1.5 mg per deciliter), and nondiabetic patients with renal insufficiency. We compared the nephrotoxic effect of an iso‐osmolar, dimeric, nonionic iodixanol with those of a low-osmolar, monomeric, nonionic iohexol. The volume of CM was about 0.8 mL/kg for each IVP procedure. The CM was injected intravenously at a rate of 2 mL/second. We hydrated the patients with 0.9% saline 1 mL/kg/hr 8–12 hours before and after IVP. The primary end point was the peak increase from baseline creatinine concentration three days after IVP. CN was defined as a ≥25% increase in serum creatinine after IVP. Late reactions were defined as those that occurred more than one hour but within seven days of completion of IVP. Serum serial creatinine will be measured before examination (day 0) and on days 2, 3, and 7. Meanwhile, the serum cytokines were also measured at day 0 (0 hr), 6 hours, and 24 hours.
Laboratory Testing
All routine examinations were performed at our clinical pathology department. Cytokines were analyzed in serum specimens by using sandwich enzyme-linked immuno-sorbent assay (ELISA) technique according to the manufacturer's instructions (IBL, Hamburg, Germany). Because of the reproducibility of this method, a change greater than 20% in the same individual was considered to be significant.
Statistical Analyses
All continuous data were tabulated and analyzed as mean ± SD. Wilcoxon rank sum test was used for within-group and between-group comparisons. Categorical data were analyzed using chi-square or Fisher's exact test, when appropriate. Differences were considered significant for a p value less than 0.05. Data were analyzed using the SAS software for Windows (Statistical Analysis System, version 9.1, SAS Institute, Cary, North Carolina, USA).
RESULTS
lists clinical characteristics of the patients receiving iodixanol or iohexol. Between the two groups (iodixanol and iohexol), only age had significant differences (p = 0.019). No association existed between the two groups, and variables such as sex, weight, height, diabetes mellitus, renal insufficiency, total water intake, previous exposure to CM, and volume of CM were not significantly different (p > 0.05).
Table 1 Clinical characteristics of the patients receiving iodixanol or iohexol group
and list serum creatinine (% change) of the patients in the iodixanol and iohexol groups. The incidence of CN was 4% (2/50) among all patients (one iodixanol, one iohexol). We found no significant differences in the incidence of CN in both groups (p = 1.0). There was no significant difference of a more than 10% change of serum creatinine in the week following IVP in both groups (p = 0.529). There was no significant difference in the serum creatinine at baseline level and days 2, 3, and 7 in both groups (p > 0.05). There was no significant difference in percentage change of serum creatinine in the week following IVP (p > 0.05).
Table 2 Change in serum creatinine on day 0–7 following intravenous pyelography
Table 3 Percentage change serum creatinine of the different groups patients receiving iodixanol or iohexol group
lists cytokines profile of the patients receiving iodixanol or iohexol. In all, 47/50 (94%) patients receiving CM did not have increased total cytokine liberation. There was no significant difference in more than 10% change of any one or total cytokines during three days in both groups (p > 0.05). There was no significant difference in the time and average level of cytokines at baseline level, 6 hours, and days 1 and 3 in both groups (p > 0.05).
Table 4 Cytokine profiles in patients receiving iodixanol or iohexol bolus injections
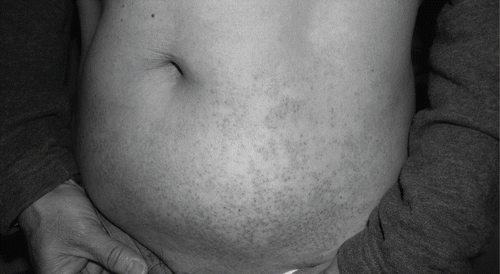
lists the allergic reactions of the patients in the iodixanol and iohexol groups. We found no significant differences in the allergic reactions in both groups (p = 0.247). The incidence of allergic reaction was 16 percent among all patients. Twelve percent (3/25) had early allergic reaction after iohexol exposure, compared to zero percent with iodixanol (p = 0.235). Twelve percent (3/25) had late reaction after iohexol exposure, compared to four percent (2/25) with iodixanol (p = 1.0). The early reaction included a burning sensation in the throat (two patients) and dizziness (one patient). The late reaction consisted mainly of skin rash. One patient had severe late adverse reaction to iodixanol and developed numerous erythematous pruritic confluent maculopapules in addition to urticaria and angioedema over upper extremities, spreading to abdominal area and groins (see ). Remission of the skin lesions was observed after one-week systemic prednisolone at 0.5 mg/kg/day. There was no significant difference in the history of previous CM examination and previous adverse reaction to CM in both groups (p > 0.05). No mortality was found.
Table 5 Early (<1 hour) and late (>1 hour to 7 days) allergic reactions in patients receiving iodixanol or iohexol bolus injections
DISCUSSION
A previous report found that the frequency of clinically important CN was 3–7%.Citation[10] The use of aggressive hydration strategies before contrast administration has been shown to be important in preventing CN.Citation[25],Citation[26] In our study, the incidence of CN was 4 percent, similar to a previous report.Citation[10] The majority of the patients were hydrated with 2830 ml of 0.9% saline during the procedure. These patients underwent IVP with less nephrotoxic contrast medium and hydration, which are important in preventing CN.
A previous report revealed that iodixanol may be slightly less nephrotoxic than iohexol.Citation[32] In our study, 8/25 (32%) patients in the iodixanol group had an increase in serum creatinine of >10% in the week following IVP, compared with 6/25 (24%) in the iohexol group (p = 0.529). However, patients in the iodixanol group were significantly older compared to those in the iohexol group (p < 0.05). These older patients in the iodixanol group did not have a higher incidence of contrast nephropathy than the iohexol group.
The previous report revealed that there is a clinically important risk of nephrotoxicity attributable to contrast material for patients with diabetes and normal renal function or for nondiabetic patients with preexisting renal insufficiency.Citation[7] In our study, 19 patients have diabetes mellitus and 29 patients have renal insufficiency. There was no significant difference in the change of serum creatinine in the week following IVP for the high-risk group receiving iodixanol or iohexol (p > 0.05). In the iodixanol group, one diabetic patient with renal insufficiency had CN. In the iohexol group, one diabetes mellitus with normal renal function had CN. The incidence of CN was 12.5 percent among our patients with diabetes mellitus and renal insufficiency, much lower than previously reported.Citation[33]
The late adverse reaction after dimeric CM administration may be explained on the basis of the presented cytokine dynamic.Citation[22] In our study, two patients with CN had only IL-6 cytokine liberation. Five patients (two iodixanol, three iohexol) had late adverse reaction, and three of them (60%) had total cytokine liberation. The majority of patients with allergic reaction had any one of cytokine liberation. The late adverse reaction of iodixanol injection had severe skin lesion. The patient had very high level of cytokine liberation.
Reactions to intravenous contrast media occur in 2–10% of investigation.Citation[30],Citation[31] Late reactions are reported with frequencies of between 8.7% and 70.0%,Citation[34–37] and reports have also suggested that there is an increased frequency of late reaction with non-ionic dimeric CM compared to non-ionic monomeric CM.Citation[31] The physiological background of late reaction is unknown. In our study, the incidence of allergic reaction was 16 percent among all patients, similar to a previous report.Citation[31] In all, 2/25 (8%) patients with iodixanol and 3/25 (12%) with iohexol had late allergic reaction, and 5/50 (10%) patients had late reaction, lower than a previous report.Citation[36] Our reports observe that iodixanol contrast medium is associated with a lower frequency of late reaction compared to iohexol, but the difference was not statistically significant (p > 0.05). Three male patients had a late reaction, different from the female predominance in a previous report. Our report was different from previous literature, but our study group is smaller and limited.
Clinically, the dimeric CM induces a lower rate of immediate reactions but has an increased risk to induce late adverse reaction.Citation[38],Citation[39] In our study, one patient had severe skin rash due to late adverse reaction after iodixanol. The skin lesion had erythema, pruritus, exanthema, edema, urticaria, and angioedema. It was initially treated as cellulites, and oral antibiotic was given with poor response. Finally, he received low dose (0.5 mg/kg) oral prednisolone therapy with resolution of skin lesion one week later.
In conclusion, new iodixanol and iohexol CM for routine IVP examination are safe and have low nephrotoxicity profile, especially in elderly and high-risk patients. Late allergic reaction may be the main adverse effect after nonionic CM infusion.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Lindholt JS. Radiocontrast induced nephropathy. Eur J Vasc Endovasc Surg. 2003; 25: 296–304
- McCullough PA, Wolyn R, Rocher LL, Levin RN, O'Neill WW. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med. 1997; 103: 368–375
- Anto HR, Chou SY, Porush JG, Shapiro WB. Infusion intravenous pyelography and renal function. Effect of hypertonic mannitol in patients with chronic renal insufficiency. Arch Intern Med. 1981; 141: 1652–1656
- Rao SR, Mieza MA, Leiter E. Renal failure in diabetes after intravenous urography. Urology. 1980; 15: 577–580
- Berg KJ. Nephrotoxicity related to contrast media. Scand J Urol Nephrol. 2000; 34: 317–322
- Weisberg LS, Kurnik PB, Kurnik BR. Risk of radiocontrast nephropathy in patients with and without diabetes mellitus. Kidney Int. 1994; 45: 259–265
- Parfrey PS, Griffiths SM, Barrett BJ, et al. Contrast material-induced renal failure in patients with diabetes mellitus, renal insufficiency, or both: A prospective controlled study. N Engl J Med. 1989; 320: 143–149
- Taliercio CP, Vlietstra RE, Ilstrup DM, et al. A randomized comparison of the nephrotoxity of iopamidol and diatrizoate in high risk patients undergoing cardiac angiography. J Am Coll Cardiol. 1991; 17: 384–390
- Moore RD, Steinberg EP, Powe NR, et al. Nephrotoxity of high-osmolality versus low-osmolality contrast media: Randomized clinical trial. Radiology. 1992; 182: 649–655
- Rudnick MR, Goldfarb S, Wexler L, et al. Nephrotoxicity of ionic and nonionic contrast media in 1196 patients: A randomized trial. The Iohexol Cooperative Study. Kidney Int. 1995; 47: 254–261
- Schwab SJ, Hlatky MA, Pieper KS, et al. Contrast nephrotoxicity: A randomized controlled trial of a nonionic and ionic radiographic contrast agent. N Engl J Med. 1989; 320: 149–153
- Barrett BJ, Parfrey PS, Vavasour HM, et al. Contrast nephropathy in patients with impaired renal function: High versus low osmolar media. Kidney Int. 1992; 41: 1274–1279
- Nicot GS. Transient glomerular proteinuria, enzymuria, and nephrotoxic reaction induced by radiocontrast media. JAMA. 1984; 252: 2432–2434
- Tervahartiala P, Kivisaari L, Kivisaari R, Virtanen I. Contrast media-induced renal morphologic lesions in diabetic rats. Acta Radiol. 1993; 34: 220–225
- Yoshioka T, Fogo A, Beckman JK. Reduced activity of antioxidant enzymes underlies contrast media-induced renal injury in volume depletion. Kidney Int. 1992; 41: 1008–1015
- Bakris GL, Lass NA, Glock D. Renal hemodynamics in radiocontrast medium induced renal dysfunction: A role for dopamine-1 receotors. Kidney Int. 1999; 56: 206–210
- Liss P, Nygren A, Erikson U, Ulfendahl HR. Injection of low and iso-osmolar contrast media decreases oxygen tension in the renal media. Kidney Int. 1998; 35: 698–702
- Anarat A, Duman N, Noyan A, Kibar M, Anarat R. The role of endothelin in radiocontrast nephropathy. Int Urol Nephrol. 1997; 29: 609–613
- Hizoh I, Haller C. Radiocontrast-induced renal tubular cell apoptosis: Hypertonic versus oxidative stress. Invest Radiol. 2002; 37: 428–434
- Beaufils H, Idee JM, Berthommier C, et al. Iobitridol, a new nonionic low-osmolality contrast agent, and iohexol: Impact on renal histology in the rat. Invest Radiol. 1995; 30: 33–39
- Gyoten M. Activation of the complement system and cytokine production by radiographic contrast media in vascular endothelial cells in vitro. Nippon Igaku Hoshasen Gakkai Zasshi. 1998; 58: 811–815, [in Japanese]
- Bohm I, Speck U, Schild H. Cytokine profiles after nonionic dimeric contrast medium injection. Invest Radiol. 2003; 38: 776–783
- Nishibori M, Kohka-Takahashi H, Mori S. Regulation of cytokine production by histamine through H2-receptor stimulation. Nippon Yakurigaku Zasshi-Folia Pharmacologica Japonica. 2001; 118: 29–35
- Robertson PW, Frewin DB, Robertson AR, et al. Plasma histamine levels following administration of radiographic contrast media. Br J Radiol. 1985; 58: 1047–1051
- Mueller C, Buerkle G, Buettner HJ, et al. Prevention of contrast media-associated nephropathy: Randomized comparison of two hydration regimens in 1620 patients undergoing coronary angioplasty. Arch Intern Med. 2002; 162: 329–336
- Erley CM, Duda SH, Rehfuss D, et al. Prevention of radiocontrast-media-induced nephropathy in patient with pre-existing renal insufficiency by hydration in combination with the adenosine antagonist theophylline. Nephrol Dial Transplant. 1999; 14: 1146–1149
- Koch JA, Plum J, Grabansee B, Modder U. Prostaglandin E1: A new agent for the prevention of renal dysfunction in high risk patients caused by radiocontrast media?. Nephrol Dial Transplant. 2000; 15: 43–49
- Lehnert T, Keller E, Gondolf K, Schaffner T, Pavenstadt H, Schollmeyer P. Effect of haemodialysis after contrast medium administration in patients with renal insufficiency. Nephrol Dial Trasplant. 1998; 13: 358–362
- Lasser EC, Lyon SG, Berry CC. Reports on contrast media reactions: Analysis of date from reports to the U.S. Food and Drug Administration. Radiology. 1997; 203: 605–610
- Moore RD, Steinberg EP, Powe NR, et al. Frequency and determinants of adverse reactions induced by high-osmolality contrast media. Radiology. 1989; 170: 727–732
- Rydberg J, Charles J, Aspelin P. Frequency of late allergy-like adverse reactions following injection of intravascular non-ionic contrast media. Acta Radiol. 1998; 39: 219–222
- Chalmers N, Jackson RW. Comparison of iodixanol and iohexol in renal impairment. Br Radiol. 1999; 72: 701–703
- Harkonen S, Kjellstrand CM. Exacerbation of diabetic renal failure following intravenous pyelography. Am J Med. 1977; 63: 939–946
- Wolf GL, Arenson RL, Cross AP. A prospective trial of ionic vs. nonionic contrast agents in routine clinical practice: Comparison of adverse effect. AJR 1989; 152: 939–944
- Sogn EE, Ødegård T, Haider T, et al. Adverse reactions following two separate intravascular injections of contrast media in the same patient. Acta Radiol. 1987; 28: 93–97
- Panto PN, Davies P. Delayed reactions to urographic contrast media. Br J Radiol. 1986; 59: 41–44
- Sutton AGC, Finn P, Grech ED, et al. Early and late reactions after the use of iopamidol 340, ioxaglate 320, and iodixanol 320 in cardiac catherization. Am Heart J. 2001; 141: 677–683
- Christiansen C, Pichler WJ, Skotland T. Delayed allergy-like reactions to x-ray contrast media: Mechanistic considerations. Eur Radiol. 2000; 10: 1965–1975
- Kanzaki T, Sakagami H. Late phase allergic reaction to a CT contrast medium (iotrolan). J Dermatol. 1991; 18: 528–531