Abstract
A 68-year-old male presented with acute myeloid leukemia, renal failure, hypokalemia, and enlarged kidneys on renal ultrasound. Renal biopsy revealed massive leukemic infiltration of the kidney. After systemic chemotherapy, the patient developed tumor lysis syndrome followed by a phase of proximal tubule dysfunction presenting as polyuria and diverse electrolyte abnormalities. In time, renal function returned to normal, as did kidney size. This report shows that renal failure, enlargement of the kidneys, and tubule dysfunction in the course of AML infiltrating the kidneys can be reversed by treatment of the hematological disease.
INTRODUCTION
Patients with leukemia are prone to renal failure. The causes are diverse and include acute tubular necrosis, leukostasis, or acute uric acid nephropathy. In addition, leukemic infiltration of the kidneys may lead to renal dysfunction.[Citation1] Autopsy studies have shown that leukemic infiltration of the kidney is often present in patients with lymphocytic leukemia. However, renal infiltration by acute myeloid leukemia (AML) is less common[Citation2–6] and mostly reported in post mortem cases[Citation2,Citation[3],Citation5] or after nephrectomy.[Citation4] Renal failure due to AML infiltrating the kidney is a rare phenomenon, and its reversibility after treatment of AML is not known.[Citation3]
We report on a case of renal failure due to massive bilateral leukemic infiltration of the kidneys in a patient with acute monocytic leukemia. To our knowledge, this is the first case describing bilateral renal sarcoma with reversibility of renal failure and tubule dysfunction after treatment of the hematological disease.
CASE REPORT
A 68-year-old Turkish male presented with a two-month history of fatigue, anorexia, weight loss, and sore, swollen gums. His medical history revealed a myocardial infarction and cardiac bypass surgery 10 years before, and type-2 diabetes mellitus seven years in duration.
Upon admission, his blood pressure was 110/65 mmHg, pulse rate was 80/min, and temperature was 37.2°C. Body weight was 70 kg, and length 1.63 m (body mass index 26.3 kg/m2). Physical examination revealed hypertrophied and easily bleeding gums.
Initial laboratory workup showed hemoglobin 8.8 g/dL, white blood cell count 19,500/μl (25% blasts, 71% monocytes), and thrombocytes 143,000/μl. Urea nitrogen was 22.9 mg/dL, creatinine 2.5 mg/dL, sodium 137 mEq/L, potassium 2.3 mEq/L, calcium 7.5 mg/dL, phosphate 2.9 mg/dL, magnesium 1.1 mg/dL, and albumin 3.7 g/dL. The creatinine value from two years before was 1.1 mg/dL. Urinalysis revealed 150 red blood cells/μl, 25 white blood cells/μl, and 1.86 g/L proteinuria. Bacterial culture of the urine was negative.
A bone marrow biopsy demonstrated 73% blasts with 10% promonocytes. Immunohistochemical staining was positive for CD14, CD36, CD15, HLA-DR, CD33, CD13, CD65, CD11b, CD64, and CD45 for 95% of the leukocytes in the bone marrow. CD34, CD117, and MPO were negative. In the peripheral blood, a myeloid population with the same immunophenotype was present that comprised 80% of the leukocytes. No Auer rods were seen in the peripheral blood smear. There were no cytogenetic abnormalities. A diagnosis of acute monocytic myeloid leukemia according to the WHO classification (FAB classification: M5a) was made.
Ultrasound showed bilaterally enlarged kidneys of 13.5 cm with a few simple cysts. There was no pathological lymphadenopathy or enlargement of liver or spleen.
Ophthalmologic investigation showed no diabetic retinopathy, nor were there any other abnormalities that can be seen in ocular involvement in AML, such as retinal hemorrhage or cotton wool spots.
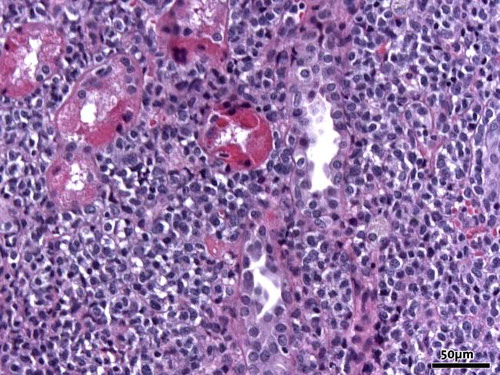
A renal biopsy was performed that showed a dense infiltrate of blasts (see ). The infiltrate pushed the tubuli apart with destruction of the architecture of both tubuli and glomeruli. The blasts were immunohistochemically consistent with the leukemic cells in the bone marrow.
Figure 1. Massive infiltration of the interstitium of the kidney by blastic myeloid cells (hematoxylin and eosin, original magnification 20×).
The patient received adequate hydration, allopurinol, and rasburicase prophylaxis and was treated for his leukemia with combination chemotherapy consisting of daunorubicin (45 mg/m2 day 1–3) and cytarabine (200 mg/ m2 day 1–7). Despite prophylaxis, he developed tumor lysis syndrome. Phosphate levels rose from 3.0 mg/dL to maximally 15.6 mg/dL, uric acid from 5.2 mg/dL to 9.2 mg/dL, and calcium levels diminished from 8.3 mg/dL to 6.2 mg/dL even with intravenous calcium suppletion. Creatinine levels initially rose to maximally 3.9 mg/dL on the third day of chemotherapy. Thereafter, renal function improved, and the plasma creatinine level stabilized at 1.1 mg/dL after approximately two weeks. Recovery of renal function went along with a phase of polyuria, hypokalemia, hypomagnesemia, hypophosphatemia, hypocalcemia, and hypouricemia, for which vigorous suppletion of fluid and electrolytes was necessary. The polyuria disappeared in the course of a few days, and fluid and electrolyte administration could be gradually tapered and stopped, after which the patient remained euvolemic and serum electrolytes stayed within normal range.
CT-scans of the abdomen on days 9 and 24 showed reduction of kidney size to 12 cm left and right.
After the first course of chemotherapy the response was evaluated and the disease was in complete remission.
DISCUSSION
In patients with leukemia, leukostasis or leukemic infiltration of the kidney may directly cause renal failure. However, leukostasis is a rare event as in a post mortem study by McKee only in 3 of 82 patients with AML leukemic thrombi or aggregates were found in the kidney.[Citation7] Also, acute renal failure due to malignant infiltration is uncommon.[Citation1] Although leukemic renal infiltration is quite often seen in post mortem studies (up to 63%),[Citation2] it does not appear to result in renal failure frequently. Most of the time, leukemic renal infiltration is seen in acute or chronic lymphocytic leukemia. The incidence of renal infiltration by acute myeloid leukemia, reported in the literature, varies between 2% to as high as 33%. This variation probably reflects different study designs, one study describing ante mortem cases,[Citation8] the other being an autopsy study.[Citation2] Extramedullary localization in myeloid leukemia, also known as granulocytic (myeloblastic) sarcoma or chloroma, most commonly involves skin, lymphoreticular structures, bone, mediastinum, epidural structures, and uterus.[Citation8] Extramedullary leukemia is especially seen in the (myelo-) monocytic form. This probably reflects the leukemic cells in these subtypes being relatively well differentiated and thereby maintaining expression of characteristics of the monocyte/macrophage system, such as extravasation and tissue invasion.
Our patient presented with gingival hyperplasia as extramedullary manifestation of acute myeloid leukemia. Upon admission, he had stage IV renal failure (estimated glomerular filtration rate according to the MDRD formula was 27 mL/min/1.73m2). The patient was known with type 2 diabetes mellitus and had proteinuria. However, the absence of diabetic retinopathy and the stable renal function prior to presentation (two years prior serum creatinine was 1.1 mg/dL and six years prior 1.0 mg/dL) argued against progressive diabetic nephropathy as the reason for deterioration of renal function. In addition, renal ultrasound revealed kidneys that were substantially enlarged, as can be seen in leukemic infiltration.[Citation3–6]
During treatment with chemotherapy, renal function deteriorated due to tumor lysis syndrome. Even though renal biopsy showed massive infiltration by leukemic cells apparently destroying renal architecture, renal function improved to the level the patient previously was known with. It has been postulated that renal failure in leukemic renal infiltration can at least in part be caused by compression of renal microvasculature and the tubule system.[Citation3] As in our patient, renal size regressed to normal with chemotherapy; one can imagine this compression to be a reversible causative factor in his renal failure. Because of the relatively low white blood cell count, leukostasis is unlikely to have played a role.[Citation7]
Both upon admission and after chemotherapy, there were remarkable electrolyte abnormalities. Hypokalemia is the electrolyte abnormality most frequently seen in patients with acute leukemia.[Citation9] Multiple factors could have caused the hypokalemia in our patient. To start, there may have been insufficient oral intake because of his malaise, which could also explain the hypomagnesemia found on admission. Besides being coincidental, the hypomagnesemia may also have contributed to the development of the hypokalemia by inducing renal potassium wasting.
Other contributing factors such as in vivo intracellular shift of potassium into rapidly proliferating leukemic cells or in vitro uptake of potassium in leukemic cells after venipuncture are less likely in this case, again because of the relatively low white blood cell count.
The activation of the renin-angiotensin-aldosterone system in the bone marrow of AML patients has been described.[Citation10] However, it remains to be seen whether this can lead to a clinically significant hyperaldosteronism with subsequent hypokalemia.
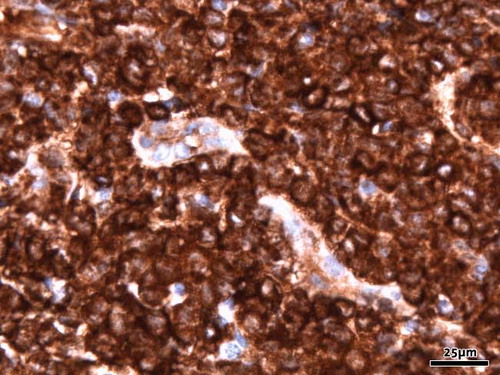
A more plausible explanation for hypokalemia combined with hypophosphatemia, hypouricemia, and polyuria is proximal renal tubule dysfunction. Some chemotherapeutic agents, especially the platinum-class drugs, are known for their ability to cause renal tubule dysfunction. However, our patient received daunorubicin and cytarabine, both of which have no such side effects. On the other hand, it has long been recognized that proximal tubule dysfunction in leukemia can be caused by lysozyme.[Citation11] The exact pathogenesis of this lysozyme-induced nephropathy to date remains obscure, and it is even possible that leukemic cell products other than lysozyme itself cause the renal tubular injury. Nevertheless, there is a distinct correlation between lysozyme levels and plasma potassium in leukemic patients. High serum lysozyme concentrations are seen particularly in M4 and M5 subtypes (FAB classification) of myeloid leukemia.[Citation12] Although we did not measure serum lysozyme concentrations or lysozymuria, the renal biopsy stained massively positive for lysozyme (see ). In our patient, supplementation of electrolytes could gradually be tapered and stopped, indicating that tubular dysfunction was temporary. One can hypothesize that as leukemic cell mass disappeared with chemotherapy, so did the high serum lysozyme and therefore its effect on the proximal tubule.
Figure 2. Immunohistochemical study of lysozym shows strong diffuse staining of the blastic cells (original magnification 40×).
To summarize, leukemic renal infiltration can frequently be seen in patients with leukemia but usually does not result in renal failure. There are few reports on acute myeloid leukemia infiltrating the kidney and causing renal failure. This report is the first to show that in such a case, successful treatment of the hematological disease can reverse renal failure, enlargement of the kidneys, and electrolyte abnormalities.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Eckman LN, Lynch EC. Acute renal failure in patients with acute leukemia. South Med J. 1978; 71(4)382–385
- Barcos M, Lane W, Gomes GA, et al. An autopsy study of 1206 acute and chronic leukemias (1958 to 1982). Cancer. 1987; 60(4)827–837
- Perazella MA, Eisen RN, Frederick WG, Brown E. Renal failure and severe hypokalemia associated with acute myelomonocytic leukemia. Am J Kidney Dis. 1993; 22(3)462–467
- Klein B, Falkson G, Simson IW, et al. Granulocytic sarcoma of the kidney in a patient with acute myelomonocytic leukemia. A case report. S Afr Med J. 1986; 70(11)696–698
- Chan YF. Granulocytic sarcoma (chloroma) of the kidney and prostate. Br J Urol. 1990; 65(6)655–656
- Bagg MD, Wettlaufer JN, Willadsen DS, Ho V, Lane D, Thrasher JB. Granulocytic sarcoma presenting as a diffuse renal mass before hematological manifestations of acute myelogenous leukemia. J Urol. 1994; 152(6 Pt 1)2092–2093
- McKee LC, Jr, Collins RD. Intravascular leukocyte thrombi and aggregates as a cause of morbidity and mortality in leukemia. Medicine (Baltimore). 1974; 53(6)463–478
- Byrd JC, Edenfield WJ, Shields DJ, Dawson NA. Extramedullary myeloid cell tumors in acute nonlymphocytic leukemia: A clinical review. J Clin Oncol. 1995; 3(7)1800–1816
- Filippatos TD, Milionis HJ, Elisaf MS. Alterations in electrolyte equilibrium in patients with acute leukemia. Eur J Haematol. 2005; 75(6)449–460
- Wulf GG, Jahns-Streubel G, Strutz F, et al. Paraneoplastic hypokalemia in acute myeloid leukemia: A case of renin activity in AML blast cells. Ann Hematol. 1996; 73(3)139–141
- Mason DY, Howes DT, Taylor CR, Ross BD. Effect of human lysozyme (muramidase) on potassium handling by the perfused rat kidney. A mechanism for renal damage in human monocytic leukemia. J Clin Pathol. 1975; 28(9)722–727
- Stark AN, Limbert HJ, Scott CS. Serum lysozyme is related to leukemic subtype in AML. Br J Hematol. 1987; 66(2)279–280