Abstract
Background. Peritonitis, the type of buffer used in the dialysate, continue ambulatory peritoneal dialysis (CAPD) of greater than two years duration, increased exposure to dialysate glucose, diabetes mellitus, and the use of beta blockers may contribute to impaired ultrafiltration. Objectives. The aim of the present study is to compare the effects of a calcium-channel blocker and a β-blocker on the peritoneal transport and clearance. Methods. We studied 48 patients with ESRD on chronic peritoneal dialysis, included 27 females and 19 males with mean age 42.6 ± 16.4 years. Two patients were excluded from the study due to peritonitis. Patients were treated either with carvedilol or lercanidipine. In all patients; peritoneal equilibration test (PET), ultrafiltration (UF), Kt/V ratio, creatinine clearance (CrCl), systolic blood pressure, diastolic blood pressure, serum BUN, creatinine, glucose, sodium, potassium, albumin, cholesterol, and triglyceride values were obtained before and after 8 weeks from the start of the drug treatment. Results. Lercanidipine and carvedilol showed a good antihypertensive effect in CAPD patients. Both drugs had a good tolerability profile and showed no effect on plasma lipids. There were no differences in terms of PET, ultrafiltration, Kt/V ratio, CrCl, systolic blood pressure, diastolic blood pressure, serum BUN, creatinine, glucose, sodium, and potassium values between both patient groups. After antihypertensive treatment, neither group showed a difference in the above-mentioned parameters (p > 0.05) except potassium, which was significantly higher in the carvedilol group (p < 0.05). Conclusions. In CAPD patients. short-term usage of carvedilol has no effect on ultrafiltration and solute transport like lercanidipine. Both drugs showed a good antihypertensive effect.
INTRODUCTION
Peritoneal dialysis (PD) is used by more than 100,000 end-stage renal disease (ESRD) patients worldwide, accounting for approximately 15% of the dialysis population.[Citation1] Functional durability of peritoneal membrane is important in long-term continue ambulatory peritoneal dialysis (CAPD) patients. Ultrafiltration (UF) failure is complication of CAPD. UF failure causes volume overload and hypertension that are the major factors in morbidity and mortality of dialysis patients. Tight control of blood pressure is of crucial importance in dialysis patients. Total incidence of impaired UF in CAPD is estimated to be between 10 and 30 percent.[Citation2] In peritoneal dialysis patients, the objective parameters for sufficiency of dialysis are urea clearance (as expressed by Kt/V) and creatinine clearance (CrCl). The status of peritoneal transportation, which is measured by peritoneal equilibration test (PET), is currently regarded as a marker for survival the CAPD patients. It is a general consensus that dialysis adequacy should have a significant impact on uremic patients' outcome. Kt/V has been used to define peritoneal dialysis adequacy. The Dialysis Outcomes Quality Initiative (K-DOQI) guideline recommended a weekly Kt/V of 2.0 for peritoneal dialysis patients.[Citation3] More recently, a minimal weekly Kt/V of 1.7 has been proposed for peritoneal dialysis patients. Initially, the target (lowest acceptable) level for total (peritoneal plus renal) Kt/V was set at 1.7 weekly, whereas that for total CrCl was set at 50 liters weekly.[Citation4]
The temporary insufficiency of UF is commonly seen in the course of peritonitis as a consequence of increased peritoneal membrane transport. On the other hand, long-term use of PD and recurrent bouts of peritonitis can end up with permanent loss of UF. Several factors may contribute to impaired UF, such as the type of buffer used in the dialysate, CAPD of greater than two years duration (even in the absence of peritonitis), increased exposure to dialysate glucose, and possibly diabetes mellitus and the use of β blockers.[Citation5–7]
β blocker drugs were reported to cause UF failure in CAPD patients.[Citation8] In these patients, hypertension, coronary artery disease, and chronic heart failure are frequently encountered diseases that necessitate β blocker usage.
Dihydropyridine and nondihydropyridine calcium channel blockers may be used safely in CAPD patients. Nifedipine is a dihydropyridine calcium channel blocker. Favaza et al. showed the effect of it on ultrafiltration in CAPD patients.[Citation9]
In this study, we aimed to compare the effects of carvedilol, a different β -blocker with both α 1 and β antagonistic effects, and lercanidipine, a dihydropyridine calcium blocker on peritoneal transport and ultrafiltration in CAPD patients.
PATIENTS AND METHODS
Patients
We studied 48 consecutive patients with ESRD on chronic peritoneal dialysis, including 27 females and 19 males with mean age 42.6 ± 16.4 years. CAPD patients used 2 L of dialysate per exchange for four exchanges daily. Patients did not have residual renal function. Informed consent was taken from patients, and hospital ethic committee approval was obtained. Baseline sociodemographic data and clinical characteristics of the patients assessed in this study are listed in . Two patients were excluded from the study due to peritonitis from both groups. Primary diagnoses of patients were shown in . Patients were treated either with carvedilol 12.5mg/d or lercanidipine 5 mg/d. Inclusion criteria as follows:
Table 1 Demographic features in groups
Table 2 Primary diagnoses in groups (p = 0.52)
all were chronic peritoneal dialysis patients;
at the time of sampling, no patient had an active infection, ongoing connective tissue disease, or immune disorders; and
patients were not on any other medications that could have affected the outcomes of this trial.
Exclusion criteria as follows:
patients with uncontrolled hypertension;
using more than two antihypertensive agents;
blood pressure greater than more than 180/110 mm/Hg;
inflammatory conditions;
volume overload;
orthostatic hypotension; and
peritonitis attacks within three months prior to this study.
In this study, antihypertensive therapy was started with study drugs after two weeks washout period for patients using other antihypertensive drugs. For both groups, no glucose concentration change was done during the study period. Antihypertensive drug usage was stopped in 7 patients using one antihypertensive drug per day and 3 patients using two different antihypertensive drugs per day. Patients waited for a two-week period without any drug for the disappearance of the drugs' effects. The tonicity of dialysis solutions used by those patients was adjusted according to their volume status. The administered dialysates were similar in both groups.
The patients in the trial were not taking any drugs, which could have an effect on the peritoneal permeability. During the trial period, the patients did not make any change in their exchange volume, exchange frequency, or tonicity of their dialysis solutions.
Method
In all patients, the following values were obtained before and after 8 weeks from the start of the drug treatment: dialysate/plasma creatinine ratio (D/P Cr) at 0, 2, and 4 hours; dialysate/dialysate 0 hour (D/Do); glucose ratios at 2 and 4 hours; UF; Kt/V ratio; CrCl; systolic blood pressure; diastolic blood pressure; HbA1c; serum BUN; creatinine; glucose; sodium; potassium; chloride; albumin; cholesterol; and triglycerides. UF volume was measured by weighing the drained dialysate. Both groups used their drugs for eight weeks. The patients with systolic blood pressure ≥140 mmHg and diastolic blood pressure ≥90 mmHg were determined as hypertensive.
All patients performed peritoneal equilibration test (PET) by Twardowski et al.[Citation10] Briefly, after an overnight exchange, peritoneal dialysis solution was drained completely while the patient remained in a sitting position for more than 20 minutes, and two liters of dialysate containing 2.27% glucose were infused intraperitoneally. After four hours, the dialysate was drained completely, and drain volume and dialysate creatinine concentrations were measured. Serum creatinine concentrations were measured on the same day. Based on the four-hour dialysate-to-plasma creatinine concentration ratio (D/Pcr), the peritoneal solute transport type was classified as low when D/Pcr was < 0.50, low-average when D/Pcr was 0.50 to 0.65, high-average when D/Pcr was 0.65 to 0.82, and high when D/Pcr was 0.82.[Citation11]
The formulas about the terms are below:
Formula 1: dialysate/plasma creatinine:
Formula 2: dialysate/plasma glucose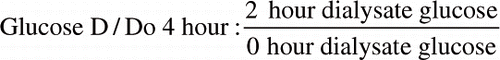
Formula 3: weekly Kt/V:
where peritoneal Kt = (24-hour dialysate urea concentration/serum urea concentration) × 24 hour drainage volume, and V = Urea distribution volume
Formula 4: urea distribution volume
Formula 5: weekly creatinine clearance
where peritoneal CrCl = daily drainage volume × (24 hour dialysate creatinine/serum creatinine)
Blood and dialysate samples were analyzed in University of Yuzuncu Yil Medical Faculty Biochemistry Laboratories with Hitachi PP modular autoanalyzer (Roche modular autoanalyzer, Tokyo, Japan) without any delay by using Roche kits, on the same day the samples were taken. Na+ was determined by ion selective electrode method (ISE). Albumin and total protein were determined by routine colorimetric methods.
Statistical Analysis
Results were evaluated by using paired and unpaired t tests and κ2 tests. p less than 0.05 is considered significant.
RESULTS
Baseline clinical characteristics are listed in and . There are no significant differences in mean age, body weight, body mass index, man-woman ratio, and CAPD duration.
Mean systolic and diastolic blood pressure of CAPD patients were 154.02 ± 25.74 mmHg and 96.54 ± 14.31 mmHg, respectively. The rate of systolic hypertension was 56.52% and diastolic hypertension was 65.21%. Thirty-three (71.8%) of the patients involved into the study had blood pressures greater than 150/90 mmHg.
Daily UF was 1468.17 ± 518.88 cc, Kt/V ratio was 2.08 ± 0.62, and CrCl was 66.31 ± 20.83 L/week. At hour 4, sD/P cr ratio and D/D0 glucose ratios were 0.64 ± 0.11 and 0.43 ± 0.10, respectively. PET analysis of the two group patients was as follows: 3 of our patients had low transport (6.5%), 24 had low-average transport (52.1%), 15 had high-average transport (32.6%), and 4 had high transport (8.6%), according to the classification by Twardowski. In the lercanidipine group, 2 of our patients had low transport (8.7%), 13 had low-moderate transport (56.6%), 6 had high-moderate transport (26%), and 2 had high transport (8.7%). In the carvedilol group, 1 of our patients had low transport (4.2%), 11 had low-moderate transport (47.8%), 7 had high-moderate transport (30.4%), and 2 had high transport (8.6%).
There were no differences in terms of D/P cr ratio at 0, 2, and 4 hours; D/Do glucose ratios at 2 and 4 hours; and UF, Kt/V ratio, CrCl, systolic blood pressure, diastolic blood pressure, serum BUN, creatinine, glucose, sodium, and potassium values between both groups (see ). After antihypertensive treatment, patients in either group did not show difference in the above parameters (p> 0.05) except potassium, which was significantly higher in the carvedilol group (p = 0.007; see ).
Table 3 Comparison of the parameters before antihypertensive treatment
Table 4 Comparison of the parameters after antihypertensive treatment
There were no significant differences between ultrafiltration and solute transport of baseline and end of treatment after eight weeks in both the lercanidipine and carvedilol groups. (UF p = 0.34, 0.07; D/Pcr p = 0.93, 0.12).
Lercanidipine and carvedilol showed a good antihypertensive effect in CAPD patients. Both drugs had a good tolerability profile and showed no effect on plasma lipids.
DISCUSSION
In our study, hypertension (>150/90 mmHg) was present in 71.8 % (33) of 46 CAPD patients, and 21% (7) of them used drugs regularly. Yilmaz et al. showed that CAPD patients had higher blood pressure rate (66%) in their study in Turkey.[Citation12] We found a higher prevalence of hypertension among CAPD patients compared with literature.[Citation13]
The incidence of ultrafiltration failure is approximately 10–40%. This defect can, in many patients, be treated with more frequent use of hypertonic solutions exchanges; however, it is severe enough to require cessation of CAPD in about 10 percent of cases.Citation[14]
Bos et al. reported a negative correlation between net ultrafiltration and blood pressure in PD patients.Citation[15] In our study, lercanidipine and carvedilol use had no significant effect on serum total protein, albumin, dialysate protein, dialysate albumin, or sodium levels after eight weeks, but patients treated with carvedilol had significantly higher serum potassium levels (p = 0.007). High potassium levels may be explained by the effect of beta blockers on Na,K-ATPase activity.[Citation16,Citation[17]]
For both drugs, no effects were found on PET and UF results before and after the therapy (p> 0.05).
Dihydropyridine and nondihydropyridine calcium channel blockers may be used safely in CAPD patients. Nifedipine is shown to lower blood pressure, but has no effect on UF in CAPD patients.[Citation9] Lercanidipine hydrochloride is a long-acting dihydropyridine calcium channel blocker. In our study, there were no significant differences between both lercanidipine and carvedilol groups due to UF and solute transport of baseline and end of treatment (UF p = 0.34, 0.07; D/Pcr p = 0.93, 0.12).
Stegmayr reported that both cardioselective and nonselective β blockers are associated with the high prevalence of ultrafiltration failure, but its mechanism is not well understood.[Citation18] Proposed mechanisms involve decline of residual renal function, development of sclerosing encapsulated peritonitis,[Citation19–22] increased glucose absorption due to increase in number and function of glucose transporters in peritoneum membrane,[Citation23] decreased portal venous pressure,[Citation24–26] increased lymphatic absorption, and increased capillary density.[Citation27,Citation[28]] In Stegmayr's study,[Citation18] ultrafiltration failure was detected in 12 patients who used β blocker (mean time of atenolol, pindolol, and metoprolol therapy = 7 months) for hypertension and atrial fibrillation. UF failure was not detected in patients not using β blocker therapy. Systolic and diastolic blood pressure, total cholesterol, triglyceride, glucose, urea, and creatinine levels were higher in patients with UF failure. UF failure recovered in the patients who discontinued β blocker therapy. The author defined that the most probable mechanism for this effect is enhanced glucose transportation from peritoneal membrane.[Citation18] Another study reported that β blocker-induced UF failure may be due to rapid decrease of osmotic solutes in dialysate.[Citation29] β blockers may reduce transcapillary UF in CAPD patients by a decrease in portal venous pressure. This effect (decreased portal venous pressure) is seen more frequent in non-selective β blockers than selective β blockers as atenolol.[Citation26] However, another study showed that the use of β blockers did not affect UF in CAPD patients.[Citation30] Animal studies revealed that α blockade with phentolamine did not influence peritoneal drainage volume.[Citation31] In one study in rabbits, α adrenergic stimulus with norepinephrine decreased urea and creatinine clearance, but the authors did not mention about drainage volume.[Citation32] β-receptor stimulus with intraperitoneal isoproterenol increased the urea and creatinine clearance in rats,[Citation33] but did not affect drainage volume in rabbits[Citation34] and dogs.[Citation35] In our study, the use of carvedilol, an antagonist of α1- and β-adrenergic receptors, had no significant effect on D/P cr ratio at 0, 2, and 4 hours; D/Do glucose ratios at 2 and 4 hours; and UF. This condition may be associated with vasodilatation due to α1 blockage effect of carvedilol.
In our study, in CAPD patients, mean Kt/V ratio was 2.08 ± 0.62 and CrCl was 66.31 ± 20.83 L/week. The patients used either lercanidipine or carvedilol did not display differences in Kt/V ratio and CrCl (p> 0.05).
In conclusion, antihypertensive treatment is frequently needed for regulation of blood pressure in CAPD patients. There were no significant differences between lercanidipine and carvedilol groups' ultrafiltration and solute transport of baseline and end of treatment. (UF p = 0.34, 0.07; D/Pcr p = 0.93, 0.12). In CAPD patients, short-term (two months) usage of carvedilol has no effect on ultrafiltration and solute transport like lercanidipine. For long-term effects, further studies are needed.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Gokal R. Taking peritoneal dialysis beyond the year 2000. Perit Dial Int. 1999; 19(Suppl. 3)35–42
- Mujais S, Nolph K, Gokal R, et al. Evaluation and management of ultrafiltration problems in peritoneal dialysis. International Society for Peritoneal Dialysis Ad Hoc Committee on Ultrafiltration Management in Peritoneal Dialysis. Perit Dial Int. 2000; 20(Suppl. 4)5–21
- NKF-K/DOQI Clinical Practice Guidelines for Peritoneal Dialysis Adequacy: Update 2000. Am J Kidney Dis. 2001; 37(1 Suppl. 1)65–136
- Lo WK, Jiang Y, Cheng SW, Cheng IK. Survival of CAPD patients in a center using three two-liter exchanges as standard regime. Perit Dial Int. 1996; 16: S163–S166
- Tattersal JE, Doyle S, Greenwood RN, Farrington K. Kinetic modelling and underdialysis in CAPD patients. Nephrol Dial Transplant. 1993; 8: 535–538
- Dubrow A, Levin NW. Biochemical and hormonal alterations in chronic renal failure. The principles and practice of nephrology2nd, HR Jacobson, GE Striker, S Klahr. Mosby, St. Louis 1995; 596–603
- Stone WJ, Hakim RM. Therapeutic options in the management of end-stage renal disease. The principles and practice of nephrology2nd, HR Jacobson, GE Striker, S Klahr. Mosby, St. Louis 1995; 650–654
- Krediet RT. Prevention and treatment of peritoneal dialysis membrane failure. Adv Renal Rep Ther. 1998; 5: 212–217
- Favazza A, Montanero D, Messa P, Antonucci F, Gropuzzo M, Mioni G. Peritoneal clearances in hypertensive CAPD patients after oral administration of clonidine, enalapril, and nifedipine. Perit Dial Int. 1992; 12: 287–291
- Twardowski ZJ, Nolph KD, Prowant B, Ryan L, Moore H, Nielsen MP. Peritoneal equilibration test. Perit Dial Bull. 1987; 7: 138–147
- Wu GG, Oreopoulos DG. Assessing peritoneal ultrafiltration and solute transport. Handbook of dialysis2nd, JT Daugirdas, TS Ing. Little, Brown and Co, Boston 1994; 328–337
- Yilmaz FM, Yilmaz G, Duranay M, et al. Cardiovascular risk factors in hemodialysis and peritoneal dialysis patients. Scand J Clin Lab Invest. 2005; 65: 739–745
- Rocco MV, Flanigan MJ, Beaver S, et al. Report from the 1995 Core Indicators for Peritoneal Dialysis Study Group. Am J Kidney Dis. 1997; 30: 165–173
- Smit W, Schouten N, van den Berg N, Langedijk MJ, Struijk DG, Krediet RT. Analysis of the prevalence and causes of ultrafiltration failure during long-term peritoneal dialysis: A cross-sectional study. Perit Dial Int. 2004; 24: 562–570
- Bos WJ, Struiyk DG, Van Olden RW, Arisal L, Krediet RT. Elevated 24-hour blood pressure in peritoneal dialysis patients with ultrafiltration failure. Adv Perit Dial. 1998; 14: 108–110
- Traub YM, Rabinov M, Rosenfeld JB, Treuherz S. Elevation of serum potassium during beta blockade. Clin Pharmacol Ther. 1980; 28: 764–768
- Rosa RM, Silva P, Young JB, et al. Adrenergic modulation of extrarenal potassium disposal. N Engl J Med. 1980; 302: 431–434
- Stegmayr BG. Beta-blockers may cause ultrafiltration failure in peritoneal dialysis patients. Perit Dial Int. 1997; 17: 541–545
- Brown P, Baddeley H, Read AE, Davies JD, McGarry J. Sclerosing peritonitis, an unusual reaction to a β-adrenergic-blocking drug (practolol). Lancet. 1974; 2: 1477–1481
- Thompson RPH, Jackson BT. Sclerosing peritonitis due to practolol. Br Med J. 1977; 1: 1393–1394
- Baxter-Smith DC, Monypenny IJ, Dorricott NJ. Sclerosing peritonitis in a patient on timolol. Lancet. 1978; 15: 149
- Clark CV, Terris R. Sclerosing peritonitis associated with metoprolol. Lancet. 1983; 23: 937
- Haimbürger O, Waniewski J, Werynski A, Tranaus A, Lindholm B. Peritoneal transport in CAPD patients with permanent loss of ultrafiltration capacity. Kidney Int. 1990; 38: 495–506
- Burroughs A, Jenkins WJ, Sherlock S, et al. Controlled trial of propranolol for the prevention of recurrent variceal hemorrhage in patients with cirrhosis. N Engl J Med. 1983; 309: 1539–1542
- Kiire CF. Controlled trial of propranolol to prevent recurrent variceal bleeding in patients with non-cirrhotic portal fibrosis. Br Med J. 1989; 298: 1363–1365
- Hillon P, Lebrec D, Munoz C, Jungers M, Goldfarb G, Benhamon J-P. Comparison of the effects of a cardioselective and a non-selective β-blocker on portal hypertension in patients with cirrhosis. Hepatology. 1982; 2: 528–531
- McHale NG, Roddie IC. The effect of intravenous adrenaline and noradenaline infusion on peripheral lymph flow in the sheep. J Physiol. 1983; 341: 517–526
- Lithell H, Pollare T, Berne C, Saltin B. The metabolic and circulatory response to beta-blockade in hypertensive men is correlated to muscle capillary density. Blood Pres. 1992; 1: 20–26
- Stegmayr B, Granbom L, Karlsson UM, Lindqvist B. Ultrafiltration failure and dialysate glucose in CAPD. Advances in peritoneal dialysis, R Khanna, KD Nolph, BF Prowant, ZJ Twardowski, DG Oreopoulos. Peritoneal Dialysis Publications, Toronto 1993; 62–64
- Selgas R, Munoz J, Cigarran S, et al. Peritoneal functional parameters after five years on continuous ambulatory peritoneal dialysis (CAPD): The effect of late peritonitis. Perit Dial Int. 1989; 9: 329–332
- Nolp KD, Ghods AJ, Van Stone J, Brown PA. The effects of intraperitoneal vasodilators on peritoneal clearances. ASAIO Trans. 1976; 22: 486–494
- Hirszel P, Lasrich M, Maher JF. Divergent effects of catecholamines on peritoneal mass transport. ASAIO Trans. 1979; 25: 110–113
- Brown EA, Kliger AS, Goffinet J, Finkelstein FO. Effect of hypertonic dialysate and vasodilators on peritoneal dialysis clearances in the rat. Kidney Int. 1978; 13: 271–277
- Maher JF, Shea C, Cassetta M, Hohnadel DC. Isoproterenol enhancement of peritoneal permeability. J Dial. 1997; 1: 319–331
- Felt J, Richard C, McCaffrey C, Levy M. Peritoneal clearance of creatinine and inulin during dialysis in dogs: Effect of splanchnic vasodilators. Kidney Int. 1979; 16: 459–469