Abstract
Background. A continuous increase in number of CKD patients entering ESRD is a growing public health threat, which reflects the present therapeutic failure usually initiating at the late stage of CKD. Objective. To study the mechanism of vascular repair in CKD patients associated with mildly impaired renal function, which included angiogenic factors such as VEFG, angiopoietin-1, and flt-1 (VEGFR1); and antiangiogenic factors such as angiopoietin-2 and KDR (VEGFR2). Results. A mild defect in angiogenic factor—namely, angiopoietin-1—was observed, whereas VEGF and flt-1 (VEGFR1) were within normal limit. Also, antiangiogenic factor—namely, angiopoietin-2—was mildly elevated, whereas KDR (VEGFR2) remained within normal limit. Conclusion. The mechanism of vascular repair appears to be adequately functional in the early stage of CKD. Therapeutic intervention at this stage can improve renal perfusion and restore renal function as indicated in normoalbuminuric, type 2 diabetic nephropathy. The authors encourage changing the conceptual view of treatment under common treatment at late stage of CKD to treatment at early stage of CKD under an environment favorable for renal regeneration.
INTRODUCTION
The American Society of Nephrology Presidential Address has recently concerned with the continuous increase in number of CKD patients entering ESRD, which appears to be a growing public health threat.[Citation1] Such a remark implies the present therapeutic failure in preventing CKD patients from entering end-stage renal disease. Under the present practice, which is in accordance with the definition of CKD defined by National Kidney Foundation K/DOQI clinical practice guidelines, CKD patients have usually been brought to specific attention of physician in particular nephrologists when creatinine clearance values are below 60 mL min/1.73 m2 (equivalent to CKD stage 3).[Citation2] This tendency to detect late-stage CKD is due to the insensitivity of the presently available diagnostic markers, such as serum creatinine determination, or microalbuminuria, which usually does not change until the actual creatinine clearance drops below the 50 percent level of normal range.[Citation3] In this regard, most of CKD patients in the early stage have been left unattended, without any specific recommendation of therapeutic or preventive strategy. There has been a general tendency for these CKD patients to gradually progress from the early stage toward late stage of CKD without any appropriate therapeutic intervention. Therefore, the actual therapeutic strategy in CKD patients is generally initiated at late stage of CKD. In fact, therapeutic response in the late stage of CKD patients at best simply slows the renal disease progression, but is unable to restore the renal function.
Two crucial issues are relevant to explain this phenomenon. First, the inappropriate therapeutic target under common practice aimed at suppression of proteinuria, or controlling of blood pressure, can't effectively prevent the progression toward end-stage renal disease. Second, we have recently demonstrated that there are multiple defects in the mechanism of vascular repair in the late stage of chronic kidney disease,[Citation4] in which treatment initiated at the late stage is unable to improve renal perfusion or function.[Citation5–10]
In contrast to the unfavorable therapeutic outcome observed in late stage of CKD patients, we as well as others have recently demonstrated that an enhanced renal perfusion, as well as a restoration of renal function, can be accomplished in treating CKD patients at an early stage (i.e., stage 1 or 2) by appropriately correcting the hemodynamic maladjustment in the renal microcirculation with multidrug vasodilators, as well as drugs that counteract the circulating toxins inducing glomerular endothelial dysfunctions, such as antioxidants, lipid-lowering substances, and anti-inflammatory drugs.[Citation11,Citation12] In order to explain such therapeutic discrepancy, we have studied the mechanism of vascular repair in the early stage of chronic kidney disease.
MATERIAL AND METHODS
Forty-five patients associated with mildly impaired renal function (creatinine clearance greater than 60 mL min/1.73 m2; average 81 ± 17 mL min/1.73 m2 versus normal 118 ± 14 mL min/1.73 m2). This value of actual creatinine clearance was in fact overestimated due to the phenomenon of hyperfiltration commonly encountered in early stage of CKD patients. Although there are no data on the underlying renal diseases in most of these 54 patients, all of them were also associated with an abnormally elevated value of fractional excretion of magnesium (4.8 ± 1.6% versus normal 1.6 ± 0.6%; p < 0.01) reflecting the presence of tubulointerstitial fibrosis, as fractional excretion of magnesium has been earlier reported to correlate with the magnitude of tubulointerstitial fibrosis.[Citation13] Therefore, the mildly impaired creatinine clearance and the elevated value of fractional excretion of magnesium observed in their patients support the status of early stage of CKD. Repeated determinations of renal function also confirmed this status. Their patients were subject to multidrug therapy, which included ACE inhibitor 10–20 mg/day, AII receptor blocker (Micardis 40–80 mg/day), calcium channel blocker (Norvasc 5–10 mg/day), antiplatelet agents (Trental 400–800 mg/day), and dipyridamole 75 mg/day, in addition to other therapeutic agents such as antioxidants (vitamin C 1000–2000 mg/day, vitamin E 400 unit/day; anti-lipid agent Lipitor 10–20 mg/day). The dosage and duration of treatment were varied in accordance with the clinical response and degree of renal functional impairment. Ages of the patients were between 27 to 70 years. Inclusion criteria include no clinical evidence of heart disease, informed consent, and no pregnancy/nursing.
Enzyme-Linked Immunosorbent Assay (ELISA) for Vascular Endothelial Growth Factor (VEGF)
This assay employs the quantative sandwich enzyme immunoassay technique. Standards and samples are pipetted into the wells, and any VEGF present is bound by the immobilized antibody. After washing away any unbound substances, an enzyme-linked polyclonal antibody specific for VEGF is added to the wells. Following a wash to remove any unbound antibody enzyme reagent, a substance solution is added to the wells, and color develops in proportion to the amount of VEGF bound in the initial step. The color development is stopped and the intensity of the color is measured.
Human Angiopoietin-1, Angiopoietin-2, FLT-1 (VEGFR1), KDR (VEGFR2) Immunoassays
These assays employ the quantitative sandwich enzyme immunoassay technique in a similar manner described previously.
Glomerular Function
A glomerular function was performed by measuring the 10 hours of endogenous creatinine clearance (CCr), and the value was converted to the body surface area of 1.73m2 by following equation:
Tubular function
Indirect tubular transport was assessed by fractional excretion of magnesium (FE Mg) through a 10-hour urinary collection, as was previously described. No diuretic was administered during or within 24 hours before the test. Briefly, after a regular supper, no additional food except drinking water ad libitum was allowed. The patients were instructed to void at 7 PM, and the urine was collected from 7 PM to 5 AM. Clotted blood from venipuncture was drawn at the end of the test for the analysis of creatinine and magnesium levels. Urine samples were analyzed the same as blood samples by the Renal Metabolic Laboratory Unit. For analysis of creatinine and magnesium, the methods described by Faulkner and King and Atomic Absorption Spectrophotometer (model 1100G; Perkin Elmer, Norwalk, Connecticut, USA) were used, respectively. A reflection of tubular transport was derived from the determination of FE Mg, which was calculated through the following formula:
Statistical analysis
Comparison of the sample mean of two quantitative variables was determined by the non-parametric method using the Mann-Whitney test. P values below 0.05 were considered to be significant.
RESULTS
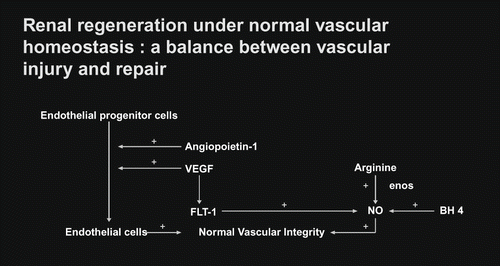
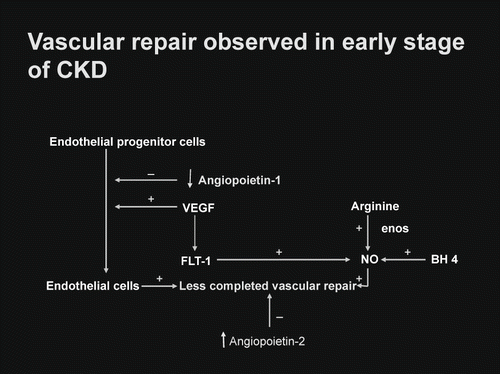
Vascular homeostasis in the early stage of chronic kidney disease was indicated in . With respective to the vascular angiogenesis, only angiopoietin-1 showed a mild defect, whereas VEGF and FLT-1 were within normal limit. With respective to the antiangiogenic factor, only angiopoietin-2 showed a mild elevation, whereas KDR (VEGFR2) was within normal limit. The mechanism of vascular repair observed in early stage of chronic kidney disease showed a mild alteration but still remained adequately functional.
Table 1 Demonstrates the mechanism of vascular repair in early stage of CKD
DISCUSSION
Vascular homeostasis is the balanced stage between vascular injury and vascular repair. Vascular injury in the renal microcirculation has been noted in a variety of clinical settings of kidney diseases.[Citation14–17] This is usually induced by circulating toxins, namely, oxidative stress (elevated reactive oxygen species in the presence of depleted antioxidants), immunocirculatory imbalance (enhanced proinflammatory cytokine tumor necrosis factor alpha, transforming growth factor beta in the presence of depleted anti-inflammatory cytokine interleukin-10), lipid disorders, glycation end products, mechanical shear stress, and other hormonal mediators.[Citation18–24] These circulating toxins are capable of inducing endothelial injury in the renal microcirculation, which can be reflected by endothelial cell cytotoxicity test or number of endothelial cells lost into the circulation.[Citation25,Citation26] An increased number of circulating endothelial cells has been noted in the early stage of CKD patients, such as diabetic nephropathy associated with normoalbuminuria, IgM mesangial nephropathy, and nephrosis with focal segmental glomerulosclerosis associated with mild reduction in creatinine clearance.[Citation27,Citation28] In response to vascular injury, vascular repair is usually initiated by recruiting the endothelial progenitor cell to the site of vascular injury, by which it would induce proliferation of endothelial cell to replace for the endothelial cell loss (see ). In this process, it also requires an adequate amount of VEGF, which would normally activate through the flt-1 (VEGFR1) in order to induce coupling of eNOS and subsequently enhance the NO production. Therefore, a normal process of vascular repair requires both endothelial cell proliferation, as well as an enhanced NO production. To accomplish a completed process of vascular repair or angiogenesis, it requires another angiogenic factor—so-called angiopoietin-1—which would strengthen the vascular repair or angiogenesis.[Citation29] A normal vascular homeostasis in the renal microcirculation would preserve an adequate perfusion to the kidney tissue. With respect to the study of vascular homeostasis observed in early stage of CKD patients, a mild degree of impairment in vascular repair as compared to the control is documented. The levels of VEGF and FLT-1 (VEGFR1) are well maintained in the early stage of CKD, which makes it likely that the activation through the classical FLT-1 pathway is adequately functional to enhance NO production (see ). The endothelial progenitor cell in this early stage of CKD appears to be intact; therefore, it can induce adequate proliferation of endothelial cell to replace for the loss. However, a mild deficiency in angiopoietin-1 level may reflect a less completeness of vascular repair in this early stage of CKD. With respect to the elevated level of antiangiogenic factor angiopoietin-2, this would be capable of inducing proliferation of vascular smooth muscle cell in the vascular wall and a plausibly progressive narrowing of vascular lumen and thereby, a progressive decline in peritubular capillary flow. Such a view concurs with the clinical observation in CKD patients who progress from early stage of CKD to late stage of CKD if the progressive decline in peritubular capillary flow is not appropriately corrected.[Citation30,Citation31] Nevertheless, the mechanism of vascular repair appears to be adequately functional in early stage of CKD. This view is supported by the clinical therapeutic strategy initiated at the early stage of CKD, in which such therapeutic approach can improve renal perfusion as well as function.[Citation11,Citation[12],Citation[31],Citation32] Thus, it is mandatory for the physician to change the conceptual view of late treatment of CKD under common practice to early treatment under an environment favorable for renal regeneration and repair. To serve this purpose, FE Mg would be a suitable diagnostic marker to screen for early stage of CKD.
ACKNOWLEDGMENTS
We are grateful to the supports of Thailand Research Fund, National Research Council Fund of Thailand, and Rachadapiseksompoj Research Grant.
REFERENCES
- BuBose TD, Jr. American Society of Nephrology Presidential Address 2006. Chronic kidney disease as a public health threat: New strategy for a growing problem. J Am Soc Nephrol 2007; 18: 1038–1045
- K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification and stratification. Am J Kidney Dis 2002; 39: 251–266
- Futrakul N, Sila-asna M, Futrakul P. Therapeutic strategy towards renal restoration in chronic kidney disease. Asian Biomedicine 2007; 1: 33–44
- Futrakul N, Butthep P, Futrakul P. Altered vascular homeostasis in chronic kidney disease. Clin Hemorheol Microcirc 2008; 38: 201–207
- Ruggennenti P, Perna A, Gherardi G, Garini G, Zoccali C, Zoccali C, et al. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet 1999; 354: 359–364
- The HOPE Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: Results of the HOPE study and MICRO-HOPE substudy. Lancet 2000; 355: 253–259
- Mogensen CE, Neldam S, Tikkanen I, Oren S, Viskoper R, Watts RE, et al. Randomized controlled trial of dual blockade of rennin-angiotensin system in patients with hypertension, microalbuminuria, and non-insulin dependent diabetes: The candesartan and lisinopril microalbuminuria (CALM) study. BMJ 2000; 321: 1440–1443
- Rossing K, Jacobsen P, Pietraszek L, Parving H-H. Renoprotective effects of adding angiotensin II receptor blocker to maximal recommended doses of ACE inhibitor in diabetic nephropathy. Diabetes Care 2003; 26: 2268–2274
- Deferrari G, Ravean M, Bersuti V, Leoncini G, Deferrari L. Optimizing therapy in the diabetic patient with renal disease: Antihypertensive treatment. J Am Soc Nephrol 2004; 15: S6–S11
- Lewis J. Increasing telmisartan vs amlodipine dose in patients with hypertension, type 2 diabetes and microalbuminuria. Nature Clinical Practice Nephrology 2007; 3: 476–477
- Futrakul N, Butthep P, Vongthavarawat V, Futrakul P, Silisalipoch S, Chaivatanarat T, et al. Early detection of endothelial injury and dysfunction in conjunction with correction of hemodynamic maladjustment can effectively restore renal function in type 2 diabetic nephropathy. Clin Hemorheol Microcirc 2006; 34: 373–382
- Futrakul N, Futrakul P, Siriviriyakul P. Correction of peritubular capillary flow reduction with vasodilators restores function in focal segmental glomerulosclerotic nephrosis. Clin Hemorheol Microcirc 2004; 31: 197–205
- Futrakul P, Yenrudi S, Futrakul N, Sensirivatana R, Watana D, Laohapaibul K, et al. Tubular function and tubulointerstitial disease. Am J Kidney Dis 1999; 33: 886–891
- Shimizu A, Kitamura H, Masuda Y, Ishizaki M, Sugisaki Y, Yamanaka N. Rare glomerular capillary regeneration and subsequent capillary regression with endothelial cell apoptosis in progressive glomerulonephritis. Am J Pathol 1997; 151: 1231–1239
- Kang DH, Kanellis J, Hugo C, Truong L, Anderson S, Kerjaschki D, et al. Role of the microvascular endothelium in progressive renal disease. J Am Soc Nephrol 2002; 13: 806–816
- Nakagawa T, Kang DH, Ohashi R, Suga S, Herrera-Acosta J, Rodriguez-Iturbe B, et al. Tubulointerstitial disease: Role of ischemia and microvascular disease. Curr Opin Nephrol Hypertens 2003; 12: 233–241
- Bohle A, Mackensen-Haen S, Wehrmann M. Significance of postglomerular capillaries in the pathogenesis of chronic renal failure. Kidney Blood Press Res 1996; 13: 191–195
- Futrakul N, Panichakul T, Butthep P, Futrakul P, Jetanalin P, Patumraj S, Siriviriyakul P. Ganoderma lucidum suppresses endothelial cell cytotoxicity and proteinuria in persistent proteinuria focal segmental glomerulosclerosis (FSGS) nephrosis. Clin Hemorheol Microcirc 2004; 31: 267–272
- Futrakul N, Tosukhowong P, Patumraj S, Siriviriyakul P, Tipprukmas N, Futrakul P. Treatments of hemodynamic maladjustment and oxidative stress prevent renal disease progression in chronically severe glomerulonephritides. Ren Fail 2003; 25: 839–844
- Wei Z, Costa K, Al-Medhi AB, Dodir C, Muzykantor V, Fisher AB. Simulated ischaemia in flow-adopted endothelial cells leads to generation of reactive oxygen species and cell signaling. Circ Res 1999; 85: 682–689
- Malek AM, Izumo J. Molecular aspects of signal transduction of shear stress in the endothelial cell. J Hypertens 1994; 12: 989–1000
- Frangos JA, Estain SG, McIntiae LV, Ives CL. Flow effects on prostacycline production by cultured human endothelial cells. Science 1984; 227: 1477–1479
- Cohen MP, Chen S, Ziyadeh FN, Shea E, Hud EA, Lautenslager GT, Sshearman CW. Evidence linking glycated albumin to altered glomerular nephrin and VEGF expression, proteinuria, and diabetic nephropathy. Kidney Int 2005; 68: 1554–1561
- Futrakul N, Sila-asna M. A default renal regeneration in chronic kidney disease. Clin Hemorheol Microcirc 2007; 36: 265–266
- Futrakul N, Panichakul T, Chaisuriya P, Sirisinha S, Patumraj S, Futrakul P. Endothelial cell cytotoxicity and renal hypoperfusion in idiopathic nephrotic syndrome. Nephron 2000; 86: 241–242
- Futrakul N, Butthep P, Chunhakarn S, Banyatsappasin W, Futrakul P, Sitprija V. A deficient VEGF enhances endothelial cell loss and impaired renal function. Ren Fail 2006; 28: 449
- Futrakul N, Butthep P, Futrakul P, Sitprija V. Improvement of renal function in type 2 diabetic nephropathy. Ren Fail 2007; 29: 155–158
- Futrakul N, Panichakul T, Sirisinha S, Futrakul P, Siriviriyakul P. Renal microvascular abnormality in chronic kidney disease. Ren Fail 2006; 28: 609–612
- Nykanen AI, Krebs R, Saaristo A, Turunen P, Alitalo K, Yla-Herttuala S, Koskinen PK, Lemstrom KB. Angiopoietin-1 protects against the development of cardiac allograft arteriosclerosis. Circulation 2003; 107: 1308–1314
- Futrakul N, Vongthavarawat V, Sirisalipoch S, Chairatanarat T, Futrakul P, Suwanwalaikorn S. Tubular dysfunction and hemodynamic alteration in normoalbuminuric type 2 diabetes. Clin Hemorheol Microcirc 2005; 32: 59–65
- Futrakul P, Sitprija V, Yenrudi S, Poshyachinda M, Sensirivatana R, Watana D, Singklwa V, Jungthiraapaanich J, Futrakul N. Glomerular endothelial dysfunction determines disease progression: A hypothesis. Am J Nephrol 1997; 17: 533–540
- Campbell R, Sangalli F, Perticucci E, Aros C, Viscarra C, Perna A, et al. Effects of combined ACE inhibitor and angiotensin II antagonist treatment in human chronic nephropathies. Kidney Int 2003; 63: 1094–1103