Abstract
Background. Acquired cystic kidney disease (ACKD) is a frequent complication in chronic hemodialysis (HD) patients and a risk factor for renal cell carcinoma. Online hemodiafiltration (HDF) provides better clearance of middle molecular weight solutes, but its effect on ACKD has not been investigated. Materials and methods. This case-control study enrolled 86 patients (43 HDF patients and 43 HD patients) who were matched according to age, sex, and duration of renal replacement therapy. The mean duration of HDF was 63 (± 35) months. The frequency and severity of ACKD was evaluated by ultrasonography using a severity scoring system. Results. We observed ACKD in 23 of the HD patients (53.5%) and 21 of the HDF patients (48.8%). This difference was not statistically significant (p = 0.829). The overall ACKD severity scores were similar in the two groups (p = 0.875). Patients on HDF had significantly lower serum levels of alkaline phosphatase and intact parathyroid hormone. Multiple logistic regression analysis indicated that duration of renal replacement therapy was the only risk factor for the presence of ACKD (p < 0.001). There was a significant correlation between duration of renal replacement therapy and ACKD severity score (r = 0.589, p < 0.001). Conclusions. Our results suggest that long-term online HDF does not reduce the frequency and severity of ACKD in dialysis patients. Duration of renal replacement therapy is the most important risk factor for ACKD. Factors that cannot be corrected by use of HDF may contribute to the formation of renal cysts.
INTRODUCTION
Acquired cystic kidney disease (ACKD) is a common and significant complication in patients undergoing long-term hemodialysis.[Citation1] Previous studies indicate that the prevalence of ACKD is more than 90% in patients who have undergone 10 years of dialysis.[Citation2] Patients with ACKD are often asymptomatic, but may experience cyst hemorrhage[Citation3] and also have a 30-fold higher risk for renal cell carcinoma.[Citation4]
The pathogenesis of ACKD may involve proliferation of tubular epithelial cells, tubular obstruction, and fluid secretion.[Citation5] It has been proposed that progressive loss of renal function and the accumulation of uremic products induces various growth factors and inflammation mediators, which may cause tubular epithelial cell proliferation and cyst formation.[Citation6–8] Primary renovascular occlusion or secondary arteriolar occlusions, which are common in end-stage kidney disease, may also be related to cyst formation.[Citation9] Factors that cause tubular obstruction, such as fibrosis, accumulation of oxalate crystals, or elevation of ß2-microglobulin, may also contribute to the development of ACKD.[Citation10]
There are no specific treatments for ACKD. Previous studies demonstrated that the prevalence of ACKD is similar in hemodialysis (HD) patients and peritoneal dialysis (PD) patients.[Citation11,Citation12] However, the clearance of middle molecular weight uremic toxins, such as ß2-microglobulin, in patients undergoing HD or PD was poor.[Citation13] Renal transplant recipients have a significantly lower prevalence and severity of ACKD, and in some cases experience regression of ACKD after transplantation.[Citation14,Citation15] This suggests that restoration of renal function may slow or reverse the progression of ACKD.
Hemodiafiltration (HDF) provides better clearance of middle molecular weight solutes by combining the convective clearance of hemofiltration and the diffusive clearance of dialysis.[Citation13,Citation16] Uremic patients treated with online HDF for longer than six months achieved a significant reduction in levels of predialysis ß2-microglobulin and advanced glycation end-product.[Citation17,Citation18]. In comparison with HD, HDF results in markedly reduced levels of proinflammatory monocyte-derived dendritic cells.[Citation19] This suggests that online HDF, which provides superior clearance of middle and large molecules, may reduce some of the complications of long-term hemodialysis. Indeed, HDF has been shown to relieve dialysis-related amyloidosis,[Citation20] carpal tunnel syndrome,[Citation21] and anemia (erythropoietin resistance) in patients undergoing long-term dialysis.[Citation22,Citation23] The effect of long-term online HDF on the development and progression of ACKD has not yet been investigated. The aims of this study were to compare the frequency and severity of ACKD in patients receiving HD and online HDF, and to identify risk factors for ACKD in patients undergoing long-term dialysis. Our study was a case-control study of 86 patients on maintenance HDF (43 patients) or HD (43 patients) and was performed in a tertiary university hospital.
SUBJECTS AND METHODS
Study Design and Subjects
The local ethics committee approved the study protocol, and we obtained informed consent from all patients. All patients were from two dialysis units of Chang Gung Memorial Hospital in Linkou and Taoyuan. There were approximately 1024 patients registered in these two dialysis units in September 2008. About 15% of these patients were receiving online HDF and the others were receiving HD. In Taiwan, thrice-weekly hemodialysis is the standard treatment covered by National Health Insurance; online HDF is provided as a patient-paid service. The choice of HDF was based on patient preference if they had been stable on hemodialysis for at least one month and had an access blood flow rate of greater than 250 mL/min. Patients who had been stable on thrice weekly HDF for at least 12 months and who agreed to receive ultrasonography examination were eligible for inclusion in this study. We excluded patients who had autosomal dominant polycystic kidney disease, a history of receiving PD or renal transplantation, or inadequate dialysis (Kt/Vurea < 1.2). We established a control group of HD patients from the computer database of all patients in the two units who were receiving regular dialysis. Each HDF patient was paired with an HD patient of the dialysis units, with each member of the pair having the same sex, comparable age (±5 years), comparable starting date for HD (within one year),[Citation24] and similar diagnostic category of ESRD (diabetic vs. non-diabetic).
Online Hemodiafiltration and Hemodialysis
The details of online HDF in our dialysis units have been described previously.[Citation17,Citation18] In brief, we performed postdilution online HDF using the Gambro AK 200 ULTRA System (Gambro, Sweden). Sterile infusate was online prepared from ultrapure dialysate that was treated with a series of three ultrafilters. Ultra-purity was insured by fortnightly sampling and culturing of dialysate, which contained less than 10 bacteria/L and undetectable endotoxin on Limulus amoebocyte assay. We performed online HDF with high-flux dialyzers (polysulphone or polymethylmethacrylate membranes) at a blood flow rate of about 300 mL/min and a dialysate flow rate of 500 mL/min. The total amount of post-dilutional infusion volume was about 20–22 L per session. HD was performed for four hours thrice-weekly using high-flux dialyzers (polysulphone, polymethylmethacrylate, or cellulose triacetate membranes) with a dialysate flow rate of 500 mL/min.
Ultrasonographic Determination of ACKD Severity
Ultrasound examinations of both kidneys of all patients were performed prospectively by an independent nephrologist to avoid inter-observer bias. ACKD was considered to be present if there were four or more cysts in each kidney.[Citation11] We modified a previously developed scoring system for polycystic kidney disease[Citation25,Citation26] to evaluate ACKD severity. We determined kidney length, size of the largest cyst, number of cysts, and the proportion of non-cystic parenchyma and then used the scoring criteria shown in .
Table 1 Acquired cystic kidney disease (ACKD) severity score, as determined by ultrasonography ACKD severity score
Clinical Biochemical Parameters and Urea Kinetics
Biochemical and hematological data, serum levels of intact parathyroid hormone (iPTH) and high-sensitivity C-reactive protein (hs-CRP) routinely checked in the dialysis units within 6 months of ultrasound examination were collected and analyzed. Urea reduction ratio (URR), delivered Kt/Vurea, and normalized protein catabolic rate (nPCR) were calculated according to standard equations.[Citation27,Citation28]
Statistical Analysis
Two separate statistical analyses were performed. The first analysis compared HD patients and HDF patients, and the second analysis compared patients with and without ACKD. Results are presented as mean ± SD unless otherwise noted. All values were tested for normal distribution using the Kolmogorov-Smirnov test; otherwise, the Mann-Whitney U test was employed. Categorical data were compared using the chi-square test. We assessed risk factors for ACKD by multivariate logistic regression analysis and examined the relationship between variables with the Pearson correlation coefficient. A p value < 0.05 was considered statistically significant.
RESULTS
Clinical Parameters
We initially identified 55 matched-pairs of patients. However, only 49 HDF patients and 46 HD patients agreed to renal ultrasonography. Thus, we enrolled a total of 86 patients (43 matched-pairs) in this study. shows the demographic data and clinical characteristics of enrolled patients. Age, gender, duration of renal replacement therapy, and etiology of renal failure (diabetic: 3 pairs; non-diabetic: 40 pairs) were not significantly different between the two groups. The frequency of ACKD at the initiation of renal replacement therapy were not significantly different in the two groups by reviewing the baseline renal images (HD group: 3.7%, HDF group: 3.1%, p = 0.903). There was comparable average duration of renal replacement therapy in the two groups (HD group: 113 ± 49 months, HDF group: 113 ± 48 months, p = 0.979). Among HDF patients, the mean duration of HDF was 63 ± 35 months (range 19 to 166 months). Patients on HDF had significantly lower levels of serum calcium (9.8 ± 0.7 mg/dL vs. 10.3 ± 0.8 mg/dL, p = 0.015), alkaline phosphatase (61 ± 17 mg/dL vs. 82 ± 64 mg/dL, p = 0.043), and iPTH (282 ± 218 mg/dL vs. 544 ± 649 mg/dL, p = 0.039), but there were no significant differences in other biochemical parameters. HDF patients had significantly higher URR (0.79 ± 0.05 vs. 0.76 ± 0.05, p = 0.018) and Kt/Vurea (1.89 ± 0.30 vs. 1.73 ± 0.27, p = 0.012).
Table 2 Patient demographic data and clinical parameters according to dialysis modalities
Frequency and Severity of ACKD
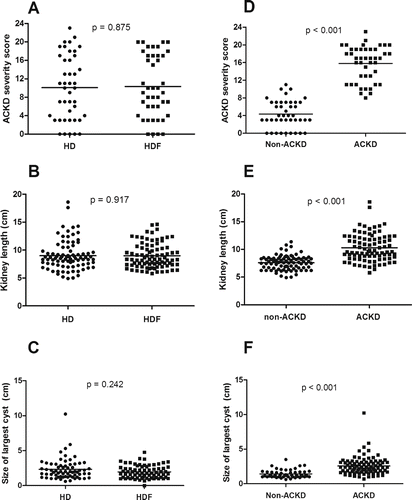
We observed ACKD in 23 of the HD patients (53.5%) and 21 of the HDF patients (48.8%). This difference was not statistically significant (p = 0.829). The overall ACKD severity scores (range 0–24) were not significantly different in the HD and HDF patients (HD: 10.1 ± 6.9, HDF: 10.3 ± 6.7, p = 0.875; see ). HD patients and HDF patients had similar average kidney length (HD: 9.0 ± 2.6 cm, vs. 9.0 ± 2.2 cm, p = 0.917; see ), and there was no significant difference in the average size of the largest cyst (HD: 2.3 ± 1.5 cm, HDF: 2.0 ± 0.9 cm, p = 0.242; see ).
Figure 1. Comparison of the severity of ACKD in patients treated with HD or online HDF and assessment of the severity of ACKD. (A) ACKD severity scores in HD patients (n = 43) and HDF patients (n = 43). (B) Kidney length in HD patients (n = 86) and HDF patients (n = 86). (C) Size of the largest cyst in HD patients (n = 86) and HDF patients (n = 86). (D) ACKD severity scores in patients with ACKD (n = 44) and without ACKD (n = 42). (E) Kidney length in ACKD patients (n = 88) and non-ACKD patients (n = 84). (F) Size of the largest cyst in ACKD patients (n = 67) and non-ACKD patients (n = 72).
Ultrasonographic Assessment of ACKD Severity
–1F show the ultrasonography assessments of ACKD severity for all patients. Patients with ACKD had significantly higher total severity scores than patients without ACKD (15.8 ± 4.0 vs. 4.4 ± 3.2, p < 0.001). The average kidney length was 7.6 ± 1.3 cm in non-ACKD patients and 10.3 ± 2.5 cm in patients with ACKD (p < 0.001). The mean size of the largest renal cyst in patients with ACKD was significantly greater than in patients without ACKD (2.6 ± 1.3 cm vs. 1.4 ± 0.6 cm, p < 0.001). None of the patients had detectable renal tumors.
Clinical Parameters of Patients with and without ACKD
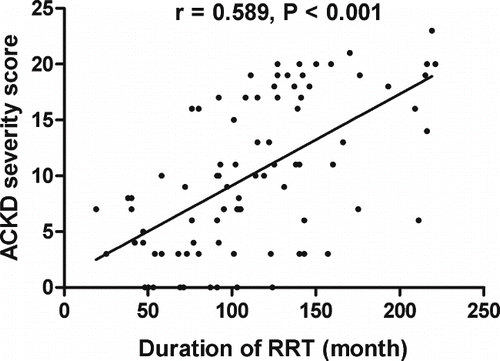
To further define risk factors for ACKD, we analyzed the clinical data of patients with and without ACKD (see ). The duration of renal replacement therapy in patients with ACKD was significantly greater than that of patients without ACKD (138 ± 42 months vs. 88 ± 41 months, p < 0.001). In addition, body mass index (22.7 ± 2.9 vs. 21.5 ± 2.4, p = 0.043) and residual glomerular filtration rate (1.4 ± 2.5 mL/min vs. 0.1 ± 0.3 mL/min, p = 0.003) were significantly lower in ACKD patients. Duration of renal replacement therapy was the only risk factor for the presence of ACKD on multivariate logistic regression analysis (p < 0.001; see ). Furthermore, there was a significant correlation between duration of renal replacement therapy and ACKD score (r = 0.589, p < 0.001; see ).
Table 3 Clinical parameters of patients with ACKD and without ACKD
Table 4 Multivariate logistic regression analysis of risk factors for ACKD
Discussion
The main result of this study is that patients on long-term online HDF have the same frequency and severity of ACKD as patients on maintenance HD. Our study provides no support for the proposal that online HDF has a clinically significant effect on modifying the course of ACKD. ACKD has become more common as the survival time of long-term dialysis patients has increased. In a previous study by our group, we have shown a significant reduction of predialysis beta-2-microglobulin levels in our HDF patients as compared with HD patients.[Citation17] Although we demonstrated that HDF also significantly reduces the level of iPTH (middle molecule) and increases the level of Kt/Vurea (small molecule) in the current study, the frequency and severity of ACKD, as measured by ultrasonography, were similar in HDF and HD patients. The result suggests that despite the HDF-mediated clearance of middle molecular weight uremic toxins, the long-term benefits of HDF on different clinical variables remain to be identified. The potential beneficial effects of long-term HDF on dialysis-related amyloidosis and survival rate are controversial.[Citation13,Citation29] Further clinical trials that measure mortality and cardiovascular complications will help us to better understand the long-term effects of HDF.[Citation13] Nevertheless, we found no harmful effects of long-term HDF in terms of risk for ACKD or renal cell carcinoma.
Our results suggest that factors other than middle molecule weight uremic toxins may contribute to the initiation and progression of ACKD. Interestingly, ACKD patients typically do not develop extra-renal cysts, suggesting that the removal of potential “cystogenic” factors does not correct intra-renal events (i.e., hyperplasia of tubular epithelium, fluid secretion, and tubular obstruction) that are responsible for the development of ACKD.[Citation5] The prevalence of ACKD in transplant patients on cyclosporine-based regimens was comparable with the prevalence of ACKD in dialysis patients.[Citation30] This indicates that other factors, such as renal ischemia, may contribute to cyst formation. As the survival time of long-term dialysis patients increases, we expect that ACKD and renal cell carcinoma will become more common. Thus, future studies are needed to clarify the mechanisms of ACKD initiation and progression and to identify effective therapies.
The results of our multivariate logistic regression analysis indicate that duration of renal replacement therapy is the most important risk factor for the presence of ACKD. Other potential risk factors, such as age and gender, were not significantly associated with the presence of ACKD. This is consistent with previous studies that have shown that the prevalence of ACKD increases as the duration of HD or PD increases.[Citation1,Citation[2],Citation[4],Citation[11],Citation12] However, none of these previous studies specifically examined the severity of ACKD in relation to the duration of dialysis in uremic patients. Our results indicate that the severity of ACKD (as measured by a scoring system) strongly correlated with the duration of renal replacement therapy. This confirms the idea that ACKD is a progressive disease and is more likely to occur as the duration of renal replacement therapy increases.
Another interesting finding of our study is that HDF patients had significantly lower levels of parathyroid hormone and alkaline phosphatase. This is in agreement with the results of Bolasco et al.,[Citation31] who demonstrated that a deceleration in bone turnover was associated with a reduction of alkaline phosphatase and iPTH after three months of HDF therapy. It is possible that HDF provides better clearance of parathyroid hormone, a middle molecular weight molecule (9500 Da).[Citation32] The effect of HDF on secondary hyperparathyroidism could also occur indirectly via the HDF-mediated enhanced removal of phosphate.[Citation33,Citation34] As we could not control for other factors that may affect serum levels of parathyroid hormone, further studies are required to clarify the hypothesis that online HDF might improve secondary hyperparathyroidism in chronic dialysis patients.
No renal cell carcinoma was detected in this prospective ultrasound screening study. A previous report showed that patients with ACKD have a higher risk of renal cell carcinoma.[Citation4] However, the screening program for ACKD and renal cell carcinoma in ESRD patients remains controversial because of the low cost-effectiveness.[Citation35] Screening for ACKD and renal cell carcinoma has been recommended for patients receiving more than three years of dialysis and for patients with unexplained hematuria.[Citation1,Citation3]
In summary, our study suggests that long-term online HDF does not reduce the frequency or severity of ACKD in patients undergoing long-term dialysis. Duration of renal replacement therapy is the most important risk factor for initiation and progression of ACKD. Factors that cannot be corrected by HDF appear to contribute to the formation of renal cysts.
REFERENCES
- Matson MA, Cohen EP. Acquired cystic kidney disease: Occurrence, prevalence, and renal cancers. Medicine (Baltimore). 1990; 69: 217–226
- Levine E, Slusher SL, Grantham JJ, Wetzel LH. Natural history of acquired renal cystic disease in dialysis patients: A prospective longitudinal CT study. Am J Roentgenol. 1991; 156: 501–506
- Moore AE, Kujubu DA. Spontaneous retroperitoneal hemorrhage due to acquired cystic kidney disease. Hemodial Int. 2007; 11((Suppl.) 3)S38–S40
- Gulanikar AC, Daily PP, Kilambi NK, Hamrick-Turner JE, Butkus DE. Prospective pretransplant ultrasound screening in 206 patients for acquired renal cysts and renal cell carcinoma. Transplantation. 1998; 66: 1669–1672
- Grantham JJ. Acquired cystic kidney disease. Kidney Int. 1991; 40: 143–152
- Oya M, Mikami S, Mizuno R, Marumo K, Mukai M, Murai M. C-jun activation in acquired cystic kidney disease and renal cell carcinoma. J Urol. 2005; 174: 726
- Konda R, Sato H, Hatafuku F, Nozawa T, Ioritani N, Fujioka T. Expression of hepatocyte growth factor and its receptor C-met in acquired renal cystic disease associated with renal cell carcinoma. J Urol. 2004; 171: 2166–2170
- Konda R, Sugimura J, Sohma F, Katagiri T, Nakamura Y, Fujioka T. Over expression of hypoxia-inducible protein 2, hypoxia-inducible factor-1alpha and nuclear factor kappaB is putatively involved in acquired renal cyst formation and subsequent tumor transformation in patients with end stage renal failure. J Urol. 2008; 180: 481–485
- Cohen EP, Elliott WC, Jr. The role of ischemia in acquired cystic kidney disease. Am J Kidney Dis. 1990; 15: 55–60
- Master U, Cruz C, Schmidt R, Dumler F, Babiarz J. Renal malignancy in peritoneal dialysis patients with acquired cystic kidney disease. Adv Perit Dial. 1992; 8: 145–149
- Park JH, Kim YO, Kim BS, et al. Comparison of acquired cystic kidney disease between hemodialysis and continuous ambulatory peritoneal dialysis. Korean J Intern Med. 2000; 15: 51–55
- Acquired cystic kidney disease in children undergoing continuous ambulatory peritoneal dialysis. Kyushu Pediatric Nephrology Study Group. Am J Kidney Dis. 1999; 34: 242–246
- van der Weerd NC, Penne EL, van den Dorpel MA, et al. Hemodiafiltration: promise for the future?. Nephrol Dial Transplant. 2008; 23: 438–443
- Ishikawa I, Yuri T, Kitada H, Shinoda A. Regression of acquired cystic disease of the kidney after successful renal transplantation. Am J Nephrol. 1983; 3: 310–314
- Schwarz A, Vatandaslar S, Merkel S, Haller H. Renal cell carcinoma in transplant recipients with acquired cystic kidney disease. Clin J Am Soc Nephrol. 2007; 2: 750–756
- Ward RA, Schmidt B, Hullin J, Hillebrand GF, Samtleben W. A comparison of on-line hemodiafiltration and high-flux hemodialysis: A prospective clinical study. J Am Soc Nephrol. 2000; 11: 2344–2350
- Lin CL, Yang CW, Chiang CC, Chang CT, Huang CC. Long-term on-line hemodiafiltration reduces predialysis beta-2-microglobulin levels in chronic hemodialysis patients. Blood Purif. 2001; 19: 301–307
- Lin CL, Huang CC, Yu CC, Yang HY, Chuang FR, Yang CW. Reduction of advanced glycation end product levels by on-line hemodiafiltration in long-term hemodialysis patients. Am J Kidney Dis. 2003; 42: 524–531
- Carracedo J, Merino A, Nogueras S, et al. On-line hemodiafiltration reduces the proinflammatory CD14+CD16+ monocyte-derived dendritic cells: A prospective, crossover study. J Am Soc Nephrol. 2006; 17: 2315–2321
- Nakai S, Iseki K, Tabei K, et al. Outcomes of hemodiafiltration based on Japanese dialysis patient registry. Am J Kidney Dis. 2001; 38: S212–S216
- Locatelli F, Marcelli D, Conte F, Limido A, Malberti F, Spotti D. Comparison of mortality in ESRD patients on convective and diffusive extracorporeal treatments. The Registro Lombardo Dialisi E Trapianto. Kidney Int. 1999; 55: 286–293
- Bonforte G, Grillo P, Zerbi S, Surian M. Improvement of anemia in hemodialysis patients treated by hemodiafiltration with high-volume on-line-prepared substitution fluid. Blood Purif. 2002; 20: 357–363
- Lin CL, Huang CC, Yu CC, et al. Improved iron utilization and reduced erythropoietin resistance by on-line hemodiafiltration. Blood Purif. 2002; 20: 349–356
- Christophe JL, van Ypersele de Strihou C, Pirson Y. Complications of autosomal dominant polycystic kidney disease in 50 hemodialysed patients. A case-control study. The U.C.L. Collaborative Group. Nephrol Dial Transplant. 1996; 11: 1271–1276
- Gabow PA, Johnson AM, Kaehny WD, Manco-Johnson ML, Duley IT, Everson GT. Risk factors for the development of hepatic cysts in autosomal dominant polycystic kidney disease. Hepatology. 1990; 11: 1033–1037
- Sung CK, Kim SH, Ahn C. Grayscale and doppler ultrasonographic findings reflecting disease severity in autosomal dominant polycystic kidney disease. J Med Ultrasound. 2008; 16: 65–73
- Daugirdas JT. The post: pre-dialysis plasma urea nitrogen ratio to estimate K.t/V and NPCR: Mathematical modeling. Int J Artif Organs. 1989; 12: 411–419
- Sargent JA. Control of dialysis by a single-pool urea model: The National Cooperative Dialysis Study. Kidney Int Suppl. 1983; S19–S25
- Canaud B, Morena M, Leray-Moragues H, Chalabi L, Cristol JP. Overview of clinical studies in hemodiafiltration: What do we need now?. Hemodial Int. 2006; 10(Suppl. 1)S5–S12
- Lien YH, Hunt KR, Siskind MS, Zukoski C. Association of cyclosporin A with acquired cystic kidney disease of the native kidneys in renal transplant recipients. Kidney Int. 1993; 44: 613–616
- Bolasco PG, Ghezzi PM, Ferrara R, et al. Effect of on-line hemodiafiltration with endogenous reinfusion (HFR) on the calcium-phosphorus metabolism: medium-term effects. Int J Artif Organs. 2006; 29: 1042–1052
- Beerenhout CH, Kooman JP, Luik AJ, Jeuken-Mertens SG, Van Der Sande FM, Leunissen KM. Optimizing renal replacement therapy—a case for online filtration therapies?. Nephrol Dial Transplant. 2002; 17: 2065–2070
- Schiffl H. Prospective randomized cross-over long-term comparison of online hemodiafiltration and ultrapure high-flux hemodialysis. Eur J Med Res. 2007; 12: 26–33
- Minutolo R, Bellizzi V, Cioffi M, et al. Postdialytic rebound of serum phosphorus: Pathogenetic and clinical insights. J Am Soc Nephrol. 2002; 13: 1046–1054
- Sarasin FP, Wong JB, Levey AS, Meyer KB. Screening for acquired cystic kidney disease: A decision analytic perspective. Kidney Int. 1995; 48: 207–219