Abstract
Vaspin, a recently identified adipokine, is a visceral adipose tissue-derived serine protease inhibitor that may have insulin sensitizing effect on adipose tissue. Herein, we measured vaspin level in patients with different stages of diabetic nephropathy (DNP), and investigated the correlation of the vaspin level with other inflammatory parameters. 106 adult type 2 diabetic patients with no known chronic inflammatory disease were included and grouped according to the stage of DNP: Albuminuria <30 mg/day and estimated glomerular filtration rate (eGFR) > 60 mL/min/1.73m2 (Group-1); albuminuria 30–300 mg/day and eGFR >60 mL/min/1.73m2 (Group-2); albuminuria >300 mL/min and eGFR <60 mL/min/1.73m2 (Group-3). Demographic, clinical and laboratory data were recorded as well as vaspin, high sensitivity C-reactive protein (hsCRP), interleukin (IL)-1 and tumor necrosis factor (TNF)-α levels. There were 38, 35 and 33 patients in Group 1, 2 and 3, respectively. Groups were similar regarding age and gender. Vaspin level did not differ between groups. When all the groups were considered, vaspin was positively correlated with IL-6 level (r = 0.215, p = 0.041). No correlation of vaspin was found with IL-1, TNF-α and hsCRP levels (p = 0.580, r = 0.054; p = 0.463, r = 0.072; p = 0.812, r = 0.025, respectively). Vaspin levels of the patients with GFR ≥60 mL/min/1.73m2 was less than that of patients with GFR <60 mL/min/1.73m2 (p = 0.03). Age and IL-6 were found to be the major determinants of vaspin level with linear regression analysis. In patients with DNP, vaspin level does not change within the early stages of DNP; while it is higher in patients with decreased GFR, which may be related with increasing inflammation regardless of the stage of the kidney disease.
Introduction
Diabetes mellitus (DM) affects many people around the world, and has been one of the most important health problems with its microvascular and macrovascular complications and increasing prevalence. It is estimated that diabetes affects 387 million people (8.3%) worldwide.Citation1 Diabetic nephropathy (DNP), the most common reason for the end stage renal disease (ESRD) in the developed countries, is the general name given to the damage in the kidneys caused by the microangiopathy.Citation2,Citation3 The primary risk factors for the development of the DNP are hyperglycemia, hypertension, the level of proteinuria, dyslipidemia, family history, glomerular filtration and the genetic factors such as the gene polymorphisms of the renin-angiotensin-aldosterone system.Citation4–8
Meanwhile, the inflammatory factors such as interleukin (IL)-1, IL-6, IL-18 and tumor necrosis factor (TNF)-α contribute to the development and the progression of the DNP and are correlated with the increased levels of albuminuria and nephropathy in the blood and the urine.Citation9 The plasma levels of IL-6, C-reactive protein (CRP), fibrinogen and serum amyloid A (SAA) predict elevated risk for progression to ESRD in the type 2 diabetic patients. It was found that the levels of CRP, SAA and IL-6 are higher in the patients with the increased glomerular basement membrane (GBM) thickness relative to the patients with normal GBM.Citation10
Vaspin, a recently identified adipokine, is a visceral adipose tissue-derived serine protease inhibitor. It was first isolated from the visceral white adipose tissues of Otsuka Long-Evans Tokushima fatty (OLETF) rat, an animal model of type 2 DM characterized by abdominal obesity, insulin resistance, hypertension, and dyslipidemia.Citation11 The presence of the reactive loop along with the beta sheets and the alpha helices suggests that vaspin belongs to the family of serpins. The studies suggested that vaspin may have insulin sensitizing effect on the white adipose tissue.Citation11,Citation12 It is thought that the increase in the vaspin level may be a potential compensatory mechanism to antagonize the unknown proteases which increase in cases of obesity and insulin resistance.Citation12 This beneficial effect has been related by Heiker et al to inhibition of kallikrein 7 by vaspin, which is a member of serine protease inhibitor family.Citation13
There is possibility that vaspin has compensatory effect in insulin resistance. Besides, there is a potential correlation of vaspin with the chronic inflammation in the diabetic patients. With these hypotheses, vaspin may have a role in the diabetic nephropathy. But the net effect on renal functions and proteinuria in patients with diabetic nephropathy at different stages has not been studied thoroughly up to now. With this background, in this study, we measured the vaspin level in patients with different stages of DNP, observed the change in the vaspin level across the DNP stages, and investigated the correlation of the vaspin level with other inflammatory parameters to reveal whether its correlation with stages of DNP, if any, is a direct correlation or a function of increased inflammation.
Methods
Our cross-sectional study was conducted on the normoalbuminuric patients and the DNP patients with different stages of DNP, who have been followed in the nephrology clinic of our hospital. The study was started after getting approval from the local ethics committee.
Only type 2 diabetic patients were included in this study. For the diagnosis of DM, guidelines of American Diabetes Association were used.Citation14 Patients with fasting glucose level higher than or equal to 126 mg/dL for at least two times or with plasma glucose level exceeding 200 mg/dL at any point in the day or in the second hour of oral glucose tolerance test (OGTT) or HbA1c level greater than or equal to 6.5% were considered to be diabetic.
Patients younger than 18 years old and older than 70 years old, patients with renal disease other than the diabetic renal disease, patients with acute kidney injury, advanced cardiovascular or respiratory disease, advanced chronic liver disease, those with positive hepatitis serology or with elevated transaminase levels twice the normal, patients with autoimmune disease, malignant diseases, a history of a systemic infectious and inflammatory condition or acute ischemic vascular disease in the last three months were excluded from the study.
Written informed consent was received from each patient selected for the study. For each participant, demographic data such as the age and gender, and height, weight, waist and hip circumference measurements were recorded. Body mass index (BMI) was calculated using the formula (BMI = weight (kg)/(height)2 (m2)). Following information is also recorded for each patient: the duration of DM, the presence of retinopathy, the presence of kidney failure, if there is kidney failure, the duration of time after the kidney failure was diagnosed. All the medicines the patients were taking were also recorded.
Venous blood samples were collected from all the patients after 12 h of fasting. The samples were kept in non-gel dry tubes and EDTA tubes. The samples were centrifuged at 1000 g for 10 min and the resulting serum and plasma samples were preserved at −80 °C till the time of the analysis. Once the collection of the samples was completed, the serum and plasma samples were defrosted and analyzed biochemically.
For all the patients, glucose, HbA1c, urea, creatinine (standardized measurement), uric acid, sodium (Na), potassium (K), calcium (Ca), phosphorus (P), total protein, albumin, parathyroid hormone (PTH), total cholesterol, HDL cholesterol, LDL cholesterol, triglyceride, aspartate transaminase (AST), alanine transaminase (ALT), ‘high sensitivity C-reactive protein’ (hsCRP), vaspin, TNF-alpha and IL-1 levels were measured and recorded. Among the hematologic parameters, hemoglobin (Hb), hematocrit, total leukocyte count, ‘mean corpuscular volume’ (MCV), thrombocyte count, iron, total iron binding capacity (TIBC), transferrin saturation and ferritin level were measured.
Glomerular filtration rate (GFR) values were estimated using the ‘Chronic Kidney Disease Epidemiology’ (CKD-EPI) formula. Proteinuria and microalbuminuria levels were computed by taking the ratio of protein and albumin amounts in the spot urine to the amount of creatinine in the spot urine. The results of these measurements were compared with the results of at least two previous measurements in the last three months, and the results which were consistent with the prior results were considered in the study. ELISA kit from Adipo Bioscience (Santa Clara, CA) was used to measure serum vaspin with the sandwich ELISA method. The same instrument and the sandwich ELISA method were used in the measurements of the serum TNF-α, IL-1 and IL-6 in addition to the ELISA method with BIOTEK EL 50 and BIOTEK EL 800 instruments (Winooski, VT).
Because of the difficulty in differentiating between stage-1 and stage-2 diabetic nephropathy proposed by the classification of Mogensen et al,Citation15 patients were divided into three groups based on their proteinuria, microalbuminuria levels and estimated GFR values:
Group 1: Patients with estimated GFR values greater than 60 mL/min/1.73m2 and with albuminuria level less than 30 mg/day based on measurements on at least two different days in the last three months (Stage 1 and 2 DNP).
Group 2: Patients with estimated GFR values greater than 60 mL/min/1.73m2 and with albuminuria level in the 30–300 mg/day range based on measurements on at least two different days in the last three months (Stage 3 DNP).
Group 3: Patients with estimated GFR values smaller than 60 mL/min/1.73m2 and with albuminuria level greater than 300 mg/day based on measurements on at least two different days in the last three months (Stage 4 DNP).
Statistical analysis was conducted by SPSS (Statistical Package for Social Sciences) for Windows 15.0 program (Chicago, IL). For the categorical variables, the descriptive statistics were the number and the percentage of cases, and for the numerical variables, the descriptive statistics were the mean and standard deviation. For pairwise group comparisons, the numerical variables with abnormal distribution, Mann–Whitney U test was used. For comparison of three groups, if the numerical variables met the normal distribution condition One Way ANOVA was used, else Kruskal–Wallis test was used. Subgroup analysis for the parametric test was done using Tukey, and for the non-parametric test using Mann–Whitney U test and the analysis was interpreted based on the Bonferroni adjustment. In the correlation analysis of the numerical variables related to vaspin normal distribution condition was not met, therefore Spearman’s correlation analysis was used. The difference of the categorical variables between the groups was evaluated using the Chi-square analysis. For the multivariate analysis of the parameters which were identified to affect the vaspin level in the univariate analysis, linear regression analysis (enter method) was used. Parameters with skewed distribution have been transformed before analysis. The alpha level for statistical significance is set at 0.05, that is a statistically significant result is one in which the observed p values is less than 0.05.
Results
Demographic data: In this study, 106 patients were included and the ratio of the number of the female to the number of male was 64/42. In Group 1, there were 38 patients with an average age of 54.2 ± 6.6 years, and number of female to number of male ratio of 24/14. In Group 2, there were 35 patients with an average age of 53.6 ± 8.1 years, and number of female to male ratio of 21/14. In Group 3, there were 33 patients with an average age of 57.6 ± 6.8 years, and number of female to male ratio of 19/14. There was no gender related difference between the groups (p = 0.890). The duration of DM in Group 1 was 8.5 ± 7.2 years, in Group 2 it was 11.8 ± 9.2 years, and in Group 3 12.2 ± 7.2 years. In terms of the duration of DM, because of the significant difference between the Groups 1 and 3 in favor of Group 3 (p = 0.012), a statistically significant difference was detected (p = 0.046).
The average BMI of the patients was 29.6 ± 5.3 kg/m2 in Group 1, 30.8 ± 5.3 kg/m2 in Group 2, and 30.7 ± 4.8 kg/m2 in Group 3, with no statistically significant difference between the groups (p = 0.131). The ratio of the waist circumference to the hip circumference in the groups was 0.93 ± 0.07, 0.94 ± 0.06, and 0.96 ± 0.06, respectively, and there was no statistically significant difference between the groups.
There was no difference between the groups in terms of the angiotensin converting enzyme inhibitor (ACEi) (p = 0.216), angiotensin receptor blocker (ARBs) (p = 0.147), acetylsalicylic acid (p = 0.754), acarbose (p = 0.148), glitazone (p = 1.000), insulin secretagogues (p = 0.011), sulfonylurea (p = 0.236) and other antidiabetic medication (p = 0.543); while the use of antihypertensive agents from other groups was less frequent in Group 2 (p = 0.026). In Group 3, metformin usage was significantly less than the other groups (p < 0.001), while diltiazem usage was significantly more frequent (p < 0.001). Insulin usage the least in Group 1, and the most frequent in Group 3. There was statistically significant difference in the insulin usage (p < 0.001). The comparison of the hematological and the biochemical parameters of the groups are presented in .
Table 1. Biochemical data of the Group 1 (GFR >60 ml/min/1.73 m2 and albuminuria <30 mg/day), Group 2 (GFR >60 ml/min/1.73 m2 and albuminuria: 30–300 mg/day) and Group 3 (GFR <60 ml/min/1.73 m2 and albuminuria >300 mg/day).
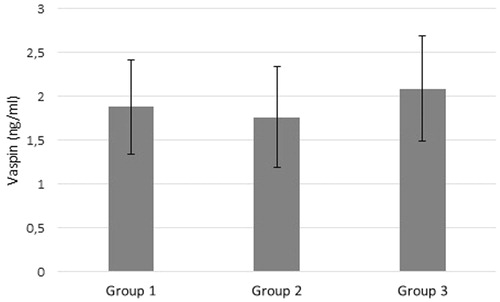
The statistics of the inflammatory indicators IL-1, TNF-α, IL-6, hsCRP and vaspin are shown in and . All inflammatory parameters except for hsCRP had a tendency to increase with the increasing stages of DNP. There was no difference between the study groups regarding vaspin level. When all the patients were divided into two groups according to GFR values, vaspin levels of the patients with GFR ≥60 mL/min/1.73m2 was less than the vaspin levels of patients with GFR <60 mL/min/1.73m2 (1.8 ± 3.3 ng/mL and 2.1 ± 3.5 ng/mL respectively; p = 0.030).
Table 2. Inflammatory markers of the Group 1 (GFR >60 ml/min/1.73 m2 and albuminuria <30 mg/day), Group 2 (GFR >60 ml/min/1.73 m2 and albuminuria: 30–300 mg/day) and Group 3 (GFR <60 ml/min/1.73 m2 and albuminuria >300 mg/day).
The analysis of the Parameters Correlated with Vaspin: When all the groups were considered, the correlation analysis of the vaspin levels and the inflammatory markers showed only a positive correlation between IL-6 and vaspin level (r = 0.215, p = 0.041). No correlation was found between the vaspin level and the IL-1, TNF-α and hsCRP levels (p = 0.580, r = 0.054; p = 0.463, r = 0.072; p = 0.812, r = 0.025, respectively). Among the other biochemical variables considered, only a weak positive correlation was observed between vaspin level and the creatinine and phosphorus levels (r = 0.199, p =0.041; r = 0.211, p = 0.030, respectively). There was no correlation of vaspin with BMI (r = 0.0.019; p = 0. 851) and waist circumference (r=-0.049; p = 0.620). The analysis also showed that the vaspin level is higher in males than in females, and the difference was almost statistically significant (2.7 ± 5.2 ng/mL, 1.4 ± 0.9 ng/mL respectively and p = 0.052). For the parameters which were identified to affect the vaspin levels during univariate analysis (age, gender, BMI, study group, GFR, hsCRP, TNF-α, IL-1, IL-6 levels), multivariate analysis was done using linear regression (enter method). Parameters with skewed distribution have been transformed before analysis. Among these parameters, only IL-6 level (B = 0.319, Beta = 0.287, p = 0.041) and age (B= −0.014, Beta= −0.262, p = 0.046) were identified as statistically significant factors determining vaspin level.
Discussion
Diabetes mellitus and chronic kidney disease are both chronic conditions characterized by extensive inflammation. As a result, several markers were used to determine the level of inflammation in the management of the patients. In CKD, inflammation is closely correlated primarily with the cardiovascular mortality and morbidity, and the presence of markers that are correlated with inflammation is important for the diagnosis and the treatment.Citation16 On the other hand, the regulatory impact of the adipocytokines on the inflammation and the vascular functions is already known.Citation17 As a result, in the recent years, there have been many studies on the adipocytokines secreted from the adipose tissue. Specifically, vaspin (a member of the serine protease inhibitor family) is an adipocytokine secreted from the visceral adipose tissue. It is also a cytokine which has been investigated in the diabetic animal models characterized by dyslipidemia with insulin resistance and hypertension.Citation11,Citation12 The presented study is the first one that explored the correlation of the serum vaspin level and the DNP stages. The basal data (age, gender, BMI, waist to hip circumference ratio) was very similar among the three patient groups. With the current knowledge, the inflammatory marker levels are expected to increase with the increasing level of renal dysfunction. As seen in , the levels of central inflammatory markers (IL-1, IL-6, TNF-α) increase as we move from Group 1–3. The levels of vaspin and hsCRP increase from Group 1 through 3, however in both cases the increase is not statistically significant (p = 0.095). In Group 3 where the GFR level is less than 60 mL/min/1.73m2, vaspin level is higher than the vaspin level of the group with GFR level more than 60 mL/min/1.73m2 (Group 1 and Group 2) (p = 0.03).
In the literature, there is no study which is directly comparable to our work. In the study of diabetic patients by Gulcelik et al.,Citation18 higher vaspin levels were found in the patients with nephropathy relative to the patients with no nephropathy. In the study by Seeger et al.,Citation19 no statistically significant difference was found between the serum vaspin concentrations of the control group (GFR >50 mL/min) and the chronic hemodialysis patients. The difference of our results from the results of this study could be explained by two factors: in our study, all the patients are diabetic, and there are no hemodialysis patients. Since vaspin is a small protein (∼50kD), it could be freely filtered by the kidneys and its level is expected to increase in the hemodialysis patients.Citation20 In another study, the vaspin levels in the hemodialysis group were smaller than the vaspin levels in the control group,Citation20 however in this study, the group with very high vaspin level (vaspin high) and the group with very low vaspin (vaspin low) were evaluated separately. In our study, we also show that there is no significant difference between the IL-6 levels of Groups 1 and 2, but both the IL-6 and the vaspin levels of the Group 3 were higher than Groups 1 and 2.
In the correlation analysis, we identified a statistically significant correlation between the IL-6 and vaspin levels (r = 0.21, p = 0.048) and a weak positive correlation between the vaspin levels the creatinine levels (r = 0.199, p = 0,041). In the literature, there is no other study which investigated the vaspin levels in patients at different stages of nephropathy. However, Seeger J et al.Citation19 investigated vaspin serum concentrations in diabetic and non-diabetic patients on chronic hemodialysis as compared with controls with GFR> 50 mL/min. Although vaspin levels were not different between dialysis patients and controls, vaspin was significantly lower in males in insulin-treated patients. They also found that vaspin was negatively associated with GFR and CRP in univariate analyzes but gender, GFR, and CRP independently predicted circulating vaspin in dialysis patients at multivariate analyzes. In the study of Inoue et al.,Citation20 when the hemodialysis and the control group patients were considered together, multivariate analysis showed a correlation between the serum vaspin level and the creatinine level. In our study, the serum vaspin level was significantly lower in the group with the higher GFR level. But no correlation was observed in multivariate analysis between vaspin and creatinine levels. Instead, vaspin was correlated with IL-6. So, the correlation with the GFR could be due to the increase in the inflammation with the decreasing GFR.
In the study by Seeger J et al,Citation19 the vaspin level in females was found higher than males. However, in our study, the vaspin level of males is higher, but the difference is not statistically significant (in females 1.4 ± 0.9, in males 2.7 ± 5.2, p = 0.052).
Another study showed a correlation between the increase in insulin resistance and the vaspin level.Citation21 This correlation cannot be investigated in our study where all the patients are diabetic, however, no difference was observed between the patients using insulin and the patients not using insulin. In our multivariate analysis of the parameters that affect the vaspin level, age and the IL 6 levels are the dominant independent parameters. Gender, BMI, GFR, hsCRP, TNF-α and IL-1 levels did not present any significant independent correlation with the vaspin level. This analysis clearly shows that the vaspin level has an important correlation with the level of IL-6, which is a major inflammatory marker. Besides, in the study by Lu et al, vaspin levels were found to be associated with beneficial outcome before and after bariatric surgery.Citation22 It also shows that this correlation is valid in the uremic diabetic group with the extensive inflammation, and this correlation is not affected by other cofactors. These result support the hypothesis that vaspin levels increase in the uremic patient groups where the insulin resistance is higher, and the vaspin could have insulin sensitizing effect on the white adipose tissue. The increase in the value of vaspin (which belongs to the serine protease inhibitor family) could also be related to the secretion of vaspin as a compensatory response to the damage in the target organ caused by proteases like alpha 1 antitrypsin. However, Hida et al.Citation11 did not show any antiprotease activity for vaspin.
The lack of a healthy control group (with only diabetes) and the low number of patients seem to be the primary shortcoming of our study. Another limitation is the fact that the correlation between vaspin and IL-6 is weak and not necessarily etiological. It could be related to decreased vaspin degradation due to decreased GFR or increased IL-6 due to decreased renal functions. The current literature does not support adequately the either way.
Conclusion
In conclusion, in patients with diabetic nephropathy, vaspin level does not change within the early stages of DNP; while it is higher in patients with decreased GFR. This increase is related with increasing inflammation regardless of the stage of the kidney disease.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- IDF Diabetes Atlas. Available at: http://www.idf.org/diabetesatlas. Accessed January 14, 2015.
- Blázquez-Medela AM, López-Novoa JM, Martínez-Salgado C. Mechanisms involved in the genesis of diabetic nephropathy. Curr Diabetes Rev. 2010;6:68–87.
- Collins AJ, Kasiske B, Herzog C, et al. Excerpts from the United States Renal Data System 2003 Annual Data Report: atlas of end-stage renal disease in the United States. Am J Kidney Dis. 2003;42(6 Suppl 5):A5–7, S1–230.
- Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412.
- Ravid M, Brosh D, Ravid-Safran D, Levy Z, Rachmani R. Main risk factors for nephropathy in type 2 diabetes mellitus are plasma cholesterol levels, mean blood pressure and hyperglycemia. Arch Intern Med. 1998;158:998–1004.
- Dahlquist G, Stattin EL, Rudberg S. Urinary albumin excretion rate and glomerular filtration rate in the prediction of diabetic nephropathy; a long-term follow-up study of childhood onset type-1 diabetic patients. Nephrol Dial Transplant. 2001;16:1382–1386.
- Pettitt DJ, Saad MF, Bennett PH, Nelson RG, Knowler WC. Familial predisposition to renal disease in two generations of Pima Indians with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1990;33:438–443.
- Hadjadj S, Tarnow L, Forsblom C, et al. Association between angiotensin-converting enzyme gene polymorphisms and diabetic nephropathy: case-control, haplotype, and family-based study in three European populations. Am Soc Nephrol. 2007;18:1284–1291.
- Navarro-González JF, Mora-Fernández C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol 2008;19:433–442.
- Dalla Vestra M, Mussap M, Gallina P, et al. Acute-phase markers of inflammation and glomerular structure in patients with type 2 diabetes. J Am Soc Nephrol 2005;16(Suppl.):S78–S82.
- Hida K, Wada J, Eguchi J, et al. Visceral adipose tissue-derived serine protease inhibitor: Aunique insulin-sensitizing adipocytokine in obesity. Proc Natl Acad Sci Unit States Am. 2005;102:10610–10615.
- Li Q, Chen R, Moriya J, et al. A novel adipocytokine, visceral adipose tissue-derived serine protease inhibitor (vaspin) and obesity. J Int Med Res. 2008;36:625–629.
- Heiker JT, Klöting N, Kovacs P, et al. Vaspin inhibits kallikrein 7 by serpin mechanism. Cell Mol Life Sci. 2013;70:2569–2583.
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;S64–S71.
- Mogensen CE, Christensen CK, Vittinghus E. The stages in diabetic renal disease. With emphasis on the stage of incipient diabetic nephropathy. Diabetes. 1983;32(Suppl.):64–78.
- Cai Q, Mukku VK, Ahmad M. Coronary artery disease in patients with chronic kidney disease: a clinical update. Curr Cardiol Rev. 2013;9:331–339.
- Carrero JJ, Cordeiro AC, Lindholm B, Stenvinkel P. The emerging pleiotrophic role of adipokines in the uremic phenotype. Curr Opin Nephrol Hypertens. 2010;19:37–42.
- Gulcelik NE, Karakaya J, Gedik A, Usman A, Gurlek A. Serum vaspin levels in type 2 diabetic women in relation to microvascular complications. Eur J Endocrinol. 2009;160:65–70.
- Seeger J, Ziegelmeier M, Bachmann A, et al. Serum levels of the adipokine vaspin in relation to metabolic and renal parameters. J Clin Endocrinol Metab. 2008;93:247–251.
- Inoue J, Wada J, Teshigawara S, et al. The serum vaspin levels are reduced in Japanese chronic hemodialysis patients. BMC Nephrol. 2012;13:163.
- Youn BS, Klöting N, Kratzsch J, et al. Serum vaspin concentrations in human obesity and type 2 diabetes. Diabetes. 2008;57:372–377.
- Lu H, Fouejeu Wamba PC, Lapointe M, et al. Increased vaspin levels are associated with beneficial metabolic outcome pre- and post-bariatric surgery. PLoS One. 2014;9:e11100223.