Abstract
Parathyroidectomy (PTx) and medical treatments are both recommended for reducing serum intact parathyroid hormone (iPTH) and curing secondary hyperparathyroidism (sHPT) in patients with chronic kidney disease (CKD), but their therapeutic effects on long-term mortality are not well-known. Thus, we aim to assess such therapeutic effect of PTx. Electronic literatures published on Pubmed, Embase, and Cochrane Central Register of Controlled Trials in any language until 27 November 2015 were systematically searched. All literatures that compared outcomes (survival rate or mortality rate) between PTx-treated and medically-treated CKD patients with sHPT were included. Finally, 13 cohort studies involving 22053 patients were included. Data were extracted from all included literatures in a standard form. The outcomes of all-cause and cardiovascular mortalities were assessed using DerSimonian and Laird’s random effects model. We find PTx-treated versus medically-treated patients had a 28% reduction in all-cause mortality and a 37% reduction in cardiovascular mortality. Thus, PTx versus medical treatments might reduce the risks of all-cause and cardiovascular mortalities in CKD patients with sHPT. Further studies with prospective and large-sample clinical trials are needed to find out the real effect of PTx and to assess whether mortality rates differ among patterns of PTx.
Introduction
Chronic kidney disease (CKD) is a worldwide public health concernCitation1 and frequently accompanied by secondary hyperparathyroidism (sHPT).Citation2 A significant risk factor for cardiovascular diseases (CVDs) in CKD patients is the accelerated vascular calcification,Citation3 but the mechanisms that account for vascular calcification under this circumstance are unknown.Citation4,Citation5 The retention of excessive P and/or Ca levels, either alone or in conjunction with recurrent episodes of hyperphosphatemia and/or hypercalcemia, may facilitate the mineral deposition in blood vessels in CKD patients. Meanwhile, parathyroid hormone (PTH) reportedly has a variety of adverse cardiovascular effects.Citation6 According to a recent assessment based on data from the U.S. Renal Data System (USRDS) registry, intact parathyroid hormone (iPTH) concentrations >495 pg/mL, versus 91–197 pg/mL, are associated with a higher risk of sudden death from CVDs.Citation7 However, very low plasma iPTH levels are also associated with excessive mortality in CKD patients at stage 5 since both high and low serum iPTH levels may induce high serum phosphorus.Citation8
Despite many advances in the medical treatments for sHPT,Citation9 there are still some patients who are nonresponsive or intolerant to pharmacological treatment. Moreover, because of high prices, relevant drugs are unavailable in some countries or regions and are inaccessible to some patients. As reported, parathyroidectomy (PTx) is superior to cinacalcet in controlling iPTH and alkaline phosphatase (ALP).Citation10 However, little evidence shows a positive effect of any of these new medical treatments on mortality.Citation11,Citation12 Moreover, routine cinacalcet therapy reduces the need for PTx in dialysis-treated adults and lowers iPTH levels, but does not improve all-cause or cardiovascular mortality.Citation13 According to a systematic review, however, cinacalcet increases risks of nausea, vomiting and hypocalcemia, suggesting harms may outweigh benefits.Citation14 In these cases, PTx is still needed by a significant proportion of dialysis patients with mineral disturbances. In the era of calcimimetics, surgery continues to play an important role in efficient control of renal HPT.Citation15,Citation16 However, most of the existing studies involve small sample sizes and only evaluate intermediate outcomes such as serum Ca and P levels, iPTH, vascular compliance and vascular calcification. These studies, individually or jointly, are not powerful enough for evaluation of mortality or cardiovascular events. This limitation makes clinicians uncertain as which type of therapies should be prescribed to CKD patients.
Considering the concerns above, we aim to assess how PTx versus medical treatments affects the mortality of CKD patients with sHPT.
Methods
Search strategy and selection criteria
The search strategy was developed and undertaken by two experienced medical librarians (Lin Chen and Kongbo Wang). They searched for clinical trials published until 27 November 2015 (date of the last search) on electronic databases PubMed, Ovid Embase (1974 to October 2015), and Wiley Cochrane Central Register of Controlled Trials (inception to October 2015). Keywords included “parathyroidectomy”, “secondary hyperparathyroidism”, “renal hyperparathyroidism”, “kidney disease”, and “dialysis”. Then one investigator (Shanlan Yu) manually searched through the references cited by relevant published reviews. We attempted to contact the authors to clarify published data if necessary.
Inclusion and exclusion criteria
Inclusion criteria were: (1) Randomized controlled trials (RCTs)/cohort/observational studies; (2) comparison of outcomes between PTx-treated and medically-treated CKD patients (irrespective of stage of CKD or type of dialysis); (3) outcomes including long-term survival rate or mortality rate (follow-up duration >12 months). Exclusion criteria were: (1) Comparison among different types of PTx or among different medical treatments; (2) kidney transplantation; (3) case reports, reviews, comments, editorial, letters, quasi experiments or unpublished studies. When more than one study from the same team or institution met the inclusion criteria, only the study with the largest sample size or the latest publication was included.
Data extraction
We selected studies and extracted data according to a standard Cochrane protocol. All the investigators independently reviewed the abstracts and identified potential articles for retrieval. Two investigators (Lin Chen and Kongbo Wang) independently reviewed the potential studies in terms of study characteristics and clinical relevance and, if appropriate, extracted data. Any disagreement between them was resolved through consensus or if necessary, by consulting the third investigator (Shanlan Yu). The following information was extracted in standardized forms: population characteristics (age, sex, race), stage and duration of CKD, presence of diabetes, and follow-up duration. We also extracted data on trial characteristics (inclusion and exclusion criteria), type of study, and trial intervention.
Quality assessment
Two investigators (Lin Chen and Kongbo Wang) independently assessed the quality of each included study using the Newcastle-Ottawa Scale (NOS).Citation17 NOS consist of three quality parameters for cohort studies: selection, comparability, and outcome, which are assigned with a maximum of four, two, and three stars, respectively. Therefore, nine stars reflect the highest quality. Studies with more than six stars are considered high quality.Citation18 Any discrepancy was resolved through a joint revaluation of the original study with a third author (Shanlan Yu).
Data synthesis
We combined dichotomous outcomes (e.g. all-cause or cardiovascular mortality) using risk ratios (RRs), odds ratios (ORs) and hazard ratios (HRs). The combined RRs (ORs or HRs) and 95% confidence intervals (CIs) were calculated separately for a homozygote comparison (PTx vs. medical treatment). Heterogeneity among studies was assessed using the I2 statistic, with I2 <25% as minimal, < 50% as moderate, and ≥50% as substantial. If heterogeneity was not substantial, a fixed-effects model was used and otherwise, a random-effects model was used. Publication bias was examined by (1) visual interpretation of funnel plot symmetry with the estimated effects plotted against standard errors, (2) Begg’s adjusted rank correlation test and (3) Egger’s regression asymmetry test. If publication bias was found, Duval and Tweedie’s trim and fill analysis was applied.
Sensitivity analyzes along with meta-regression were conducted to assess whether heterogeneity could be attributed to measurable sources. Subgroup analyzes based on the mean follow-up duration and deadline of inclusion were both performed to identify possible causes of heterogeneity and assess the robustness of the relationships. The mean follow-up duration was categorized as <36 months and ≥36 months. Since sHPT control is much more efficient than cinacalcet (together with VDRA) and since studies published before 2005 definitely excluded the cinacalcet-treated patients (cinacalcet became available in US and Europe in 2004), we set the criteria for deadline of inclusion at the year 2004. Since significant heterogeneity was observed in our study (I2 = 70.3%), we combined the data from enrolled trials and assessed all-cause mortality using DerSimonian and Laird’s random effects model. All analyzes were performed on STATA 12.0 (STATA College Station, TX). p < 0.05 was considered significant except for the publication bias test (p < 0.10).
Results
Selection and characteristics of studies
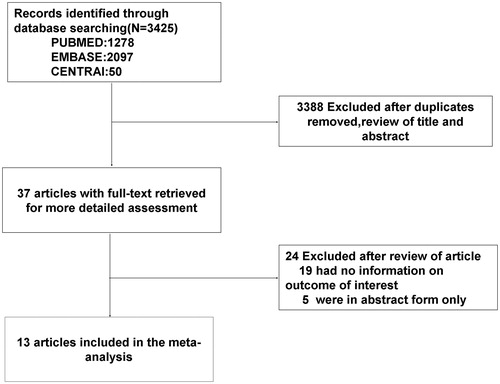
A total of 3425 potential studies were identified. Of them, 37 studies were retrieved for detailed evaluation, and finally 24 studies were excluded due to absence of information on the outcomes of interest (19 articles) or provision of data only in abstract form (5 articles). Finally, 13 studiesCitation19–31 fulfilled the eligibility criteria (), with 10052 PTx-treated patients and 12001 medically-treated patients (totally 22053 patients). The 13 studies were all cohort studies with sample sizes from 20 to 4558 patients, and reported prevalence rates of diabetes from 0% to 44.3%. The patients (mean age 52.9 years old) received 40.2–150.5 months of dialysis and were followed up for 12–96 months. All studies included patients on dialysis. Most studies adjusted a wide range of potential confounders to investigate the association between PTx and all-cause mortality, including age, sex, body mass index (BMI), diabetes, duration of dialysis, and dialysis modality. Twelve studies presented data with all-cause mortalityCitation19–29,Citation31 and six studies with cardiovascular mortality.Citation19,Citation20,Citation23,Citation26,Citation28,Citation30 lists the key characteristics of the 13 studies.
Table 1. Detailed characteristics of studies included in the meta-analysis.
Quality assessment
The 13 studies had an average NOS score of 7.31, and were all of high quality (NOS score ≥6) except one study.Citation31
Heterogeneity and publication bias
The between-study heterogeneity is substantial in the analysis of all-cause mortality (I2 = 70.3%, p < 0.001) and is 5.8% in the analysis of cardiovascular mortality. To further explore the heterogeneity for all-cause mortality, we performed sensitivity analyzes, in which one trial was excluded at a time from the pooled effects to determine whether it was particularly influential. The exclusion of any of three studiesCitation22,Citation24,Citation29 did not significantly alter the overall risk estimate (HR 0.71, 95% CI 0.60–0.84), and the heterogeneity disappeared (I2 = 36.0%, p = 0.13). Meanwhile, the exclusion of any of two studiesCitation22,Citation29 slightly altered the overall risk estimate (HR 0.75, 95% CI 0.65–0.86), but the heterogeneity also disappeared (I2 = 38.2%, p = 0.10).
We also used meta-regression to explore whether any of the sources of heterogeneity could explain the inconsistency. Specifically, we assessed the relationship between PTx and all-cause mortality against mean age, race, diabetes mellitus (%), gender (% female), sample size (N < 200 vs. N ≥ 200), mean follow-up duration and deadline of inclusion. However, no factor was found significantly contributive to heterogeneity (p > 0.1 for each), indicating any of these characteristics could well explain the between-trial heterogeneity.
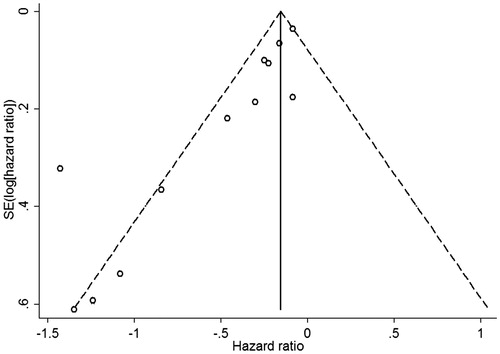
We observed significant asymmetry of funnel plots () and significant evidence of publication bias in both Begg’s test and Egger’s tests (both p =0.003). When we explored the influence of a potential publication bias using the trim-and-fill method, no potential missing data were replaced, and findings were generally similar with a decreased risk of all-cause mortality in the PTx-treated patients (HR 0.72, 95% CI 0.62–0.84).
Effect of PTx versus medical treatments on mortality
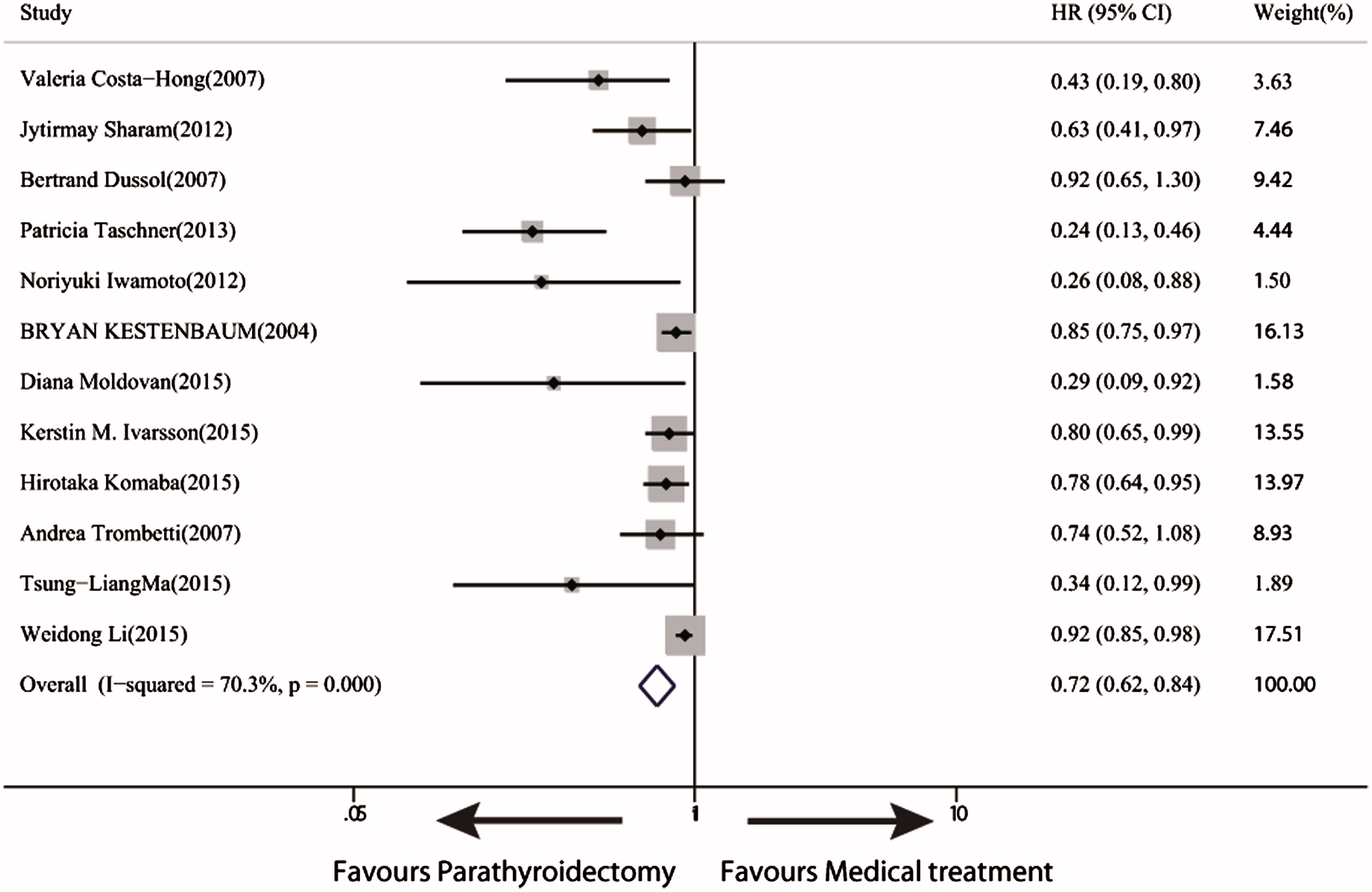
Twelve trials with 22003 participants reported all-cause mortality, and the follow-up duration ranged from 12 to 96 months. We noted a significant reduction in all-cause mortality (28%) in PTx-treated patients compared with the medically-treated patients (HR 0.72, 95% CI 0.62–0.84) (. Six trials with 10615 patients reported cardiovascular mortality (HR 0.63, 95% CI, 0.48–0.83).
Effect of PTx versus medical treatments on mortality against follow-up duration
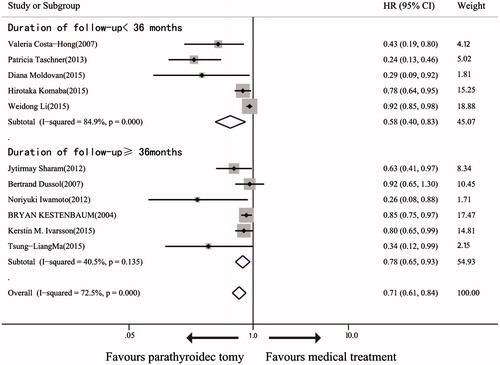
We examined the 11 trialsCitation19–26,Citation28,Citation29,Citation31 that investigated the effect of PTx versus medical treatment on mortality against follow-up duration (<36 vs. ≥36 months). In the six trials with follow-up duration <36 months, the mortality rates are significantly different between PTx-treated and medically-treated patients (.
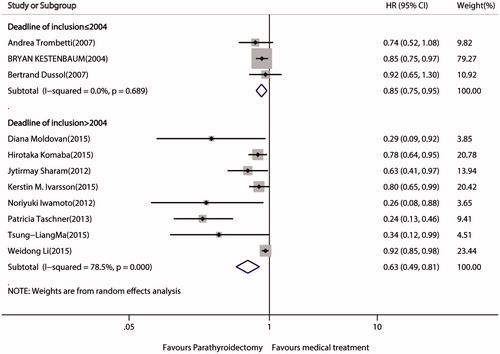
Effect of PTx versus medical treatments on mortality against deadline of inclusion
We examined the 11 trials that investigated the effect of PTx versus medical treatment on mortality against deadline of inclusion (≤2004 vs. > 2004). The relative benefit of PTx versus medical treatment is consistent for deadline of inclusion ≤2004 (z = 2.87, p = 0.004) with no heterogeneity and for deadline >2004 (Z = 3.63, p < 0.001) with significant heterogeneity (I2 = 78.5%, p < 0.001) (.
Discussion
This systematic review involving 22053 patients shows a 28% reduction in all-cause mortality and a 37% reduction in cardiovascular mortality following PTx (total PTx with or without autoimplantation, subtotal PTx) versus medical treatments. The post-PTx survival improvement is most likely attributed to multifactorial causes. High (and very low) iPTH levels, accompanied by high Ca and P levels, are associated with higher cardiovascular morbidity and mortality.Citation32 Experiments show that a high iPTH level will increase cellular calcium uptake, promote fibrosis of cardiac myocytes,Citation33,Citation34 and stimulate the development of atherosclerosis.Citation35 Clinical studies reveal that a high iPTH level promotes aortic valve calcification and causes myocardial injuries, including cardiac hypertrophy, myocardial calcification and myocardial fibrosis.Citation36–38 PTx, through reducing serum Ca and P levels, can dramatically reduce iPTH levels, better controls serum minerals and simultaneously suppress extraosseous calcification stress.Citation39 These favorable changes in mineral metabolism are the most proximate mechanisms underlying that PTx may improve survival and cardiovascular outcomes. Moreover, left ventricular function in patients with advanced sHPT and dilated cardiomyopathy was markedly improved by PTx.Citation40 Other possible mechanisms for post-PTx survival improvement include reduction in fracture risk;Citation41 improvement in anemia and hypertension,Citation42 cardiac autonomic nervous system,Citation43 life-threatening condition of calciphylaxis,Citation44 immune system,Citation45 muscle strength,Citation46 as well as sustained reduction in fibroblast growth factor 23.Citation47 PTx may lower long-term mortality by avoiding the exposure to medical therapy that is prescribed to attenuate iPTH and phosphate excess.
Subgroup analyzes were conducted to find out whether all-cause mortality rates differed among follow-up durations and among deadline of inclusion. As for follow-up durations, a-priori subgroup analysis shows a significant decrease in all-cause mortality following PTx versus medical treatment in the trails with follow-up durations <36 months. It is indicated that the effect of PTx versus medical treatment on mortality reduction is found with relatively longer follow-up duration (12–36 months). As for deadline of inclusion, a significant decrease in all-cause mortality following PTx versus medical treatment is found irrespective of deadline of inclusion (≤2004 and >2004), which indicates that the survival benefit after PTX did not disappear after the emergence of cinacalcet in US and Europe in 2004.
The heterogeneity in HRs is not unexpected given the differences in both study designs and populations. For example, only patients (including the control group) with indication for PTx were enrolled,Citation22 but diabetic patients were excluded.Citation19 Significant between-study heterogeneity was observed in the analysis of all-cause mortality (I2 = 70.3%, p < 0.001). The exclusion of any of two studiesCitation22,Citation29 did not significantly alter the overall risk estimate (HR 0.75, 95% CI 0.65–0.86), and the heterogeneity also disappeared (I2 = 38.2%, p = 0.10). The incorporative HRs for all-cause mortality of the whole trials was 0.72, while HRs were reportedly 0.24Citation22 and 0.92.Citation29 The HRs were low in the data given by Goldenstein et al.Citation22 and high in Li et al.Citation29 because the patients in Goldenstein et al.Citation22 were more symptomatic, severely ill and had the indication for PTx. Moreover, the patients submitted to PTx were younger (46 vs. 50 years old) and less frequently diabetic (5.6% vs. 15.6%) compared to those who did had no chance to undergo surgery.Citation22 Moreover, the higher HR in Li et al.Citation29 is attributed to the relatively shorter follow-up and dialysis durations compared to other studies.
To draw the conclusion, we should point out the higher short-term mortality and rehospitalization rate after PTx according to a previous study.Citation48 Meanwhile, PTx has contraindications and probably some severe complications, such as bilateral palsy of recurrent laryngeal nerves.Citation49,Citation50 Cinacalcet reduces serum iPTH levels by binding selectively to the calcium-sensing receptor in parathyroid tissues and enhances the inhibitory effect of extracellular calcium ions on PTH secretion even under normal or low serum calcium concentrations. Thus, cinacalcet may be the first choice for patients in whom sHPT is hardly manageable by PTx.Citation51
This systematic review has several strengths, including the broad search strategy with standard Cochrane protocols and a large sample size. To our knowledge, this is the first systematic review and meta-analysis to compare the long-term effect of PTx versus medical treatment on CKD patients with sPTH.
This review also has some limitations. First, only cohort studies were included. Second, one major problem is the “comparator group” in all the cohort studies: patients who had the indication for PTX but either declined or were unsuitable for surgery suffered a higher mortality risk (because of ongoing severe sHPT and its clinical consequences or high burden of co-morbidities) – confounding by indication! Even multivariate adjustments could never correct for all the clinical reasons for declining the surgical procedure. Third, the difference of mortality among different types of PTx was not assessed, though they reportedly have similar clinical outcomes in a present studyCitation31 and a Meta-Analysis,Citation52 which was different with another Meta-Analysis.Citation53 Fourth, none of the studies formally assessed adverse events, and follow-up was short—although this would bias in favor of finding a decrease in mortality over time. As probably expected, a moderate degree of heterogeneity was noted between studies. Studies with smaller sample size (N < 200) were also enrolled in the meta-analysis, but these studies might, very likely, lack sufficient statistical power to reveal the true association. Moreover, our funnel plot analysis showed a potential publication bias, which is difficult to ascertain. It is implied that our findings might overestimate the true effect if we miss non-significant studies.
In summary, our systematic review shows that PTx versus medical treatment might decrease long-term mortality in CKD patients with sHPT. Nevertheless, further reviews with prospective and large-sample clinical trials are needed to find out the real effect of PTx and to assess whether mortality rates differ among patterns of PTx.
Funding information
This research received no specific financial support.
Acknowledgements
The authors would like to thank Ya Liu and Zuoguang Wang in the Department of Hypertension Research for collecting data. We appreciate the help of Zhuguang Wen for providing review and grammatical refinement of the manuscript.
Disclosure statement
The authors declare no conflict of interest.
References
- Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379:165–180.
- Cunningham J, Locatelli F, Rodriguez M. Secondary hyperparathyroidism: Pathogenesis, disease progression, and therapeutic options. Clin J Am Soc Nephrol. 2011;6:913–921.
- Liabeuf S, Desjardins L, Diouf M, et al. The addition of vascular calcification scores to traditional risk factors improves cardiovascular risk assessment in patients with chronic kidney disease. PLoS One. 2015;10:e0131707–26181592.
- Felsenfeld AJ, Levine BS, Rodriguez M. Pathophysiology of calcium, phosphorus, and magnesium dysregulation in chronic kidney disease. Semin Dial. 2015;28:564–577.
- Shanahan CM. Mechanisms of vascular calcification in CKD-evidence for premature ageing. Nat Rev Nephrol. 2013;9:661–670.
- Chertow GM, Correa-Rotter R, Block GA, et al. Baseline characteristics of subjects enrolled in the Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events (EVOLVE) trial. Nephrol Dial Transplant. 2012;27:2872–2879.
- Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK. Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol. 2001;12:2131–2138.
- Avram MM, Mittman N, Myint MM, Fein P. Importance of low serum intact parathyroid hormone as a predictor of mortality in hemodialysis and peritoneal dialysis patients: 14 years of prospective observation. Am J Kidney Dis. 2001;38:1351–1357.
- Fukagawa M, Komaba H, Kakuta T. Hyperparathyroidism in chronic kidney disease patients: An update on current pharmacotherapy. Expert Opin Pharmacother. 2013;14:863–871.
- Ghani A, Baxter P. Surgical parathyroidectomy versus cinacalcet therapy: in the management of secondary hyperparathyroidism. Otolaryngol Head Neck Surg. 2012;146:220–225.
- Chertow GM, Block GA, Correa-Rotter R, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367:2482–2494.
- Palmer SC, Teixeira-Pinto A, Saglimbene V, et al. Association of drug effects on serum parathyroid hormone, phosphorus, and calcium levels with mortality in CKD: A meta-analysis. Am J Kidney Dis. 2015;66:962–971.
- Parfrey PS, Drueke TB, Block GA, et al. The effects of cinacalcet in older and younger patients on hemodialysis: The evaluation of cinacalcet HCl therapy to lower cardiovascular events (EVOLVE) trial. Clin J Am Soc Nephrol. 2015;10:791–799.
- Ballinger AE, Palmer SC, Nistor I, Craig JC, Strippoli GF. Calcimimetics for secondary hyperparathyroidism in chronic kidney disease patients. Cochrane Database Syst Rev. 2014;12:CD006254.
- Lorenz K, Bartsch DK, Sancho JJ, Guigard S, Triponez F. Surgical management of secondary hyperparathyroidism in chronic kidney disease-a consensus report of the European society of endocrine surgeons. Langenbecks Arch Surg. 2015;400:907–927.
- Yuan CM, Nee R, Narayan R, Abbott KC. Treatment of secondary hyperparathyroidism with parathyroidectomy instead of cinacalcet: time to pick the low-hanging fruit. Am J Kidney Dis. 2012;60:179–181.
- Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012.
- Gu WJ, Wang F, Tang L, Liu JC. Single-dose etomidate does not increase mortality in patients with sepsis: A systematic review and meta-analysis of randomized controlled trials and observational studies. Chest. 2015;147:335–346.
- Costa-Hong V, Jorgetti V, Gowdak LH, Moyses RM, Krieger EM, De Lima JJ. Parathyroidectomy reduces cardiovascular events and mortality in renal hyperparathyroidism. Surgery. 2007;142:699–703.
- Sharma J, Raggi P, Kutner N, et al. Improved long-term survival of dialysis patients after near-total parathyroidectomy. J Am Coll Surg. 2012;214:400–407.
- Dussol B, Morand P, Martinat C, et al. Influence of parathyroidectomy on mortality in hemodialysis patients: A prospective observational study. Ren Fail. 2007;29:579–586.
- Goldenstein PT, Elias RM, de Freitas do Carmo LP, et al. Parathyroidectomy improves survival in patients with severe hyperparathyroidism: A comparative study. PLoS One. 2013;8:e68870.
- Iwamoto N, Sato N, Nishida M, et al. Total parathyroidectomy improves survival of hemodialysis patients with secondary hyperparathyroidism. J Nephrol. 2012;25:755–763.
- Kestenbaum B, Andress DL, Schwartz SM, et al. Survival following parathyroidectomy among United States dialysis patients. Kidney Int. 2004;66:2010–2016.
- Ivarsson KM, Akaberi S, Isaksson E, et al. The effect of parathyroidectomy on patient survival in secondary hyperparathyroidism. Nephrol Dial Transplant. 2015;30:2027–2033.
- Komaba H, Taniguchi M, Wada A, Iseki K, Tsubakihara Y, Fukagawa M. Parathyroidectomy and survival among Japanese hemodialysis patients with secondary hyperparathyroidism. Kidney Int. 2015;88:350–359.
- Trombetti A, Stoermann C, Robert JH, et al. Survival after parathyroidectomy in patients with end-stage renal disease and severe hyperparathyroidism. World J Surg. 2007;31:1014–1021.
- Ma TL, Hung PH, Jong IC, et al. Parathyroidectomy is associated with reduced mortality in hemodialysis patients with secondary hyperparathyroidism. Biomed Res Int. 2015;2015:639587.
- Li W, Zhang M, Du S, et al. Impact of parathyroidectomy on survival among hemodialysis patients: A prospective cohort study. Nephrology (Carlton). 2016;21:133–138.
- Conzo G, Perna AF, Savica V, et al. Impact of parathyroidectomy on cardiovascular outcomes and survival in chronic hemodialysis patients with secondary hyperparathyroidism. A retrospective study of 50 cases prior to the calcimimetics era. BMC Surg. 2013;13:S4–S24268127.
- Moldovan D, Racasan S, Kacso IM, et al. Survival after parathyroidectomy in chronic hemodialysis patients with severe secondary hyperparathyroidism. Int Urol Nephrol. 2015;47:1871–1877.
- Tentori F, Wang M, Bieber BA, et al. Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: the DOPPS study. Clin J Am Soc Nephrol. 2015;10:98–109.
- Zhang YB, Smogorzewski M, Ni Z, Massry SG. Altered cytosolic calcium homeostasis in rat cardiac myocytes in CRF. Kidney Int. 1994;45:1113–1119.
- Amann K, Ritz E, Wiest G, Klaus G, Mall G. A role of parathyroid hormone for the activation of cardiac fibroblasts in uremia. J Am Soc Nephrol. 1994;4:1814–1819.
- Rashid G, Bernheim J, Green J, Benchetrit S. Parathyroid hormone stimulates the endothelial nitric oxide synthase through protein kinase A and C pathways. Nephrol Dial Transplant. 2007;22:2831–2837.
- Raggi P, Boulay A, Chasan-Taber S, et al. Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease. J Am Coll Cardiol. 2002;39:695–701.
- Soares AA, Freitas WM, Japiassu AV, et al. Enhanced parathyroid hormone levels are associated with left ventricle hypertrophy in very elderly men and women. J Am Soc Hypertens. 2015;9:697–704.
- Leibowitz D. Left ventricular hypertrophy and chronic renal insufficiency in the elderly. Cardiorenal Med. 2014;4:168–175.
- Hamano T, Matsui I, Mikami S, et al. Fetuin-mineral complex reflects extraosseous calcification stress in CKD. J Am Soc Nephrol. 2010;21:1998–2007.
- Goto N, Tominaga Y, Matsuoka S, et al. Cardiovascular complications caused by advanced secondary hyperparathyroidism in chronic dialysis patients; special focus on dilated cardiomyopathy. Clin Exp Nephrol. 2005;9:138–141.
- Rudser KD, de Boer IH, Dooley A, Young B, Kestenbaum B. Fracture risk after parathyroidectomy among chronic hemodialysis patients. J Am Soc Nephrol. 2007;18:2401–2407.
- Coen G, Calabria S, Bellinghieri G, et al. Parathyroidectomy in chronic renal failure: short- and long-term results on parathyroid function, blood pressure and anemia. Nephron. 2001;88:149–155.
- Zhang J, Yu X, Sun B, et al. Parathyroidectomy and heart rate variability in patients with stage 5 CKD. Clin J Am Soc Nephrol. 2013;8:1378–1387.
- Girotto JA, Harmon JW, Ratner LE, Nicol TL, Wong L, Chen H. Parathyroidectomy promotes wound healing and prolongs survival in patients with calciphylaxis from secondary hyperparathyroidism. Surgery. 2001;130:645–650.
- Yasunaga C, Nakamoto M, Matsuo K, Nishihara G, Yoshida T, Goya T. Effects of a parathyroidectomy on the immune system and nutritional condition in chronic dialysis patients with secondary hyperparathyroidism. Am J Surg. 1999;178:332–336.
- Chou FF, Lee CH, Lee CT. Muscle force and bone mineral density after parathyroidectomy and subcutaneous autotransplantation for secondary hyperparathyroidism. World J Surg. 1999;23:452–456. discussion 456–457.
- Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–4408.
- Ishani A, Liu J, Wetmore JB, et al. Clinical outcomes after parathyroidectomy in a nationwide cohort of patients on hemodialysis. Clin J Am Soc Nephrol. 2015;10:90–97.
- Tominaga Y, Matsuoka S, Uno N, Sato T. Parathyroidectomy for secondary hyperparathyroidism in the era of calcimimetics. Ther Apher Dial. 2008;12:S21–S26.
- Tominaga Y. Basic and clinical aspects of calcimimetics. Calcimimetic is indicated for patients with surgically uncontrollable hyperparathyroidism. Clin Calcium. 2008;18:67–71.
- Rodríguez M, Goodman WG, Liakopoulos V, Messa P, Wiecek A, Cunningham J. The use of calcimimetics for the treatment of secondary hyperparathyroidism: A 10 year evidence review. Semin Dial. 2015;28:497–507.
- Chen J, Zhou QY, Wang JD. Comparison between subtotal parathyroidectomy and total parathyroidectomy with autotransplantation for secondary hyperparathyroidism in patients with chronic renal failure: A meta-analysis. Horm Metab Res. 2015;47:643–651.
- Jia X, Wang R, Zhang C, Cui M, Xu D. Long-term outcomes of total parathyroidectomy with or without autoimplantation for hyperparathyroidism in chronic kidney disease: A meta-analysis. Ther Apher Dial. 2015;19:477–485.