Abstract
Background: Intravenous (IV) iron supplementation is widely used in hemodialysis (HD) patients to treat their periodic losses. However, the ideal dose and frequency is unknown. The goal of the study is to see if a 20 mg dose of iron IV at the end of each session of HD as iron maintenance is better than the iron prior therapy. We analyze the erythropoiesis activity (EA) and functional iron (FI) after four weeks of treatment.
Methods: In 36 patients, we measure reticulocyte count and content of hemoglobin reticulocyte (CHr) as EA and FI markers, respectively, before and after the treatment. Before the study, 23 patients received another different therapy with IV iron as maintenance therapy.
Results: Reticulocyte count: 49.7 ± 23.8 × 103 before and 47.2 ± 17.2 × 103 after the treatment (p= 0.51). The CHr: 34.8 ± 3.7 pg and 34.4 ± 3.5 pg, respectively, (p= 0.35), showing an excellent correlation with the other FI markers (serum iron r = 0.6; p = 0.001; saturation transferrin r = 0.49; p = 0.004); that is not shown with the serum ferritin (r = 0.23; p = 0.192) or the hepcidin levels (r = 0.22; p = 0.251). There was not a correlation between the C-Reactive Protein, reticulocyte count, and CHr. The 13 patients who did not receive the iron prior to the study showed high FI levels, but not an increased of the serum ferritin or the serum hepcidin levels.
Conclusions: The administration of a small quantity of iron at the end of every HD session keeps the EA and the FI levels and allows reducing the iron overload administered and/or decreasing the iron stores markers in some patients.
Introduction
Appropriate anemia management for hemodialysis (HD) patients is changing. Deficiencies of erythropoietin and iron play a role in their genesis, and both must be corrected. Optimal hemoglobin (Hb) target and strategies to balance erythropoiesis-stimulating agents (ESAs) and intravenous (IV) iron administration remain unclear.Citation1 ESAs dose has decreased since recent studies have reported adverse outcomes of effective anemia correction with ESAs.Citation2–4 On the other hand, iron supplementation is widely used in HD patients to treat iron deficiency, prevent its development in ESA-treated patients, reduce the ESAs dose and raise the Hb levels in the presence or absence of ESAs treatment, so its use has been increased last years.Citation1,Citation5 However, despite iron supply routine, nephrologists have not yet reached consensus on several questions: What is the best strategy for iron therapy in dose or frequency terms and whether the best way to administer iron is consistently (i.e., weekly to monthly) or sporadically.Citation6,Citation7 Also, there is not a consensus on the treatment of anemia by the functional iron deficiency (FID); in fact, most recent guidelinesCitation8,Citation9 recommend caution with the routine IV iron administration. However, recent studies say that some of those patients may improve with IV iron therapy.Citation10 There is also no evidence of defining any specific upper limits for serum ferritin levels. However, it is not recommended (usually limits 500–800 ng/mL) for IV iron therapy.Citation8 Because of that, there is a variation in IV iron therapy in different countries and over the time.Citation11 The goal of the this paper was to verify whether the administration of 20 mg iron sucrose IV in each HD session was enough for increasing erythropoietic activity, as measured by reticulocyte counts, and improving functional iron, as measured by content of hemoglobin reticulocyte (CHr) after four weeks of maintenance therapy.
Methods
Study design and patients
In order to include a sufficient number of patients in the study, all patients of our HD unit were assessed for this study. We recruited 50 patients who had been receiving intermittent HD (3 times a week for 3–4 h) for 12 weeks. Patients that had any kind of infections (defined by the presence of fever or antibiotics treatments) or that received red blood cells transfusion during the study or weeks before, and those patients with higher ferritin levels (≥1400 mg/mL) were not included. A total of 36 patients, 13 women and 23 men, were included.
Before starting our study, 23 patients were receiving 100 mg of IV sucrose iron diluted in 100 mL of saline in the last half an hour of HD, as maintenance therapy. Eight of them once a week, nine every two weeks and six once a month. The 13 remaining patients did not receive the iron prior to the study.
Interventions
During the study, we administered 20 mg of iron sucrose at the end of every HD session. Following the directions of our Pharmacy Service, the syringe with 1 mL of iron, in most cases iron sucrose originator, is diluted in 10 mL of saline out of the treatment room and then is injected into each patient. The iron was injected for 1 min through the venous line before the disconnection, as set out in the drug details of the product.Citation12 Every patient remained in the HD unit for at least 30 min after they finished the iron treatment. During the study, the dose of ESAs was unchanged. The patients provided their informed consent.
Data collection and determinations
The study consisted in the basal collection of venous blood samples in the second and fourth week of treatment. The basal data and the data collected at the end of the study were hematimetria [hemoglobin (Hb), hematocrit (Htc), red blood cells (RBC) and reticulocyte count], and iron status [serum iron, serum transferrin, transferrin saturation (TSAT), serum ferritin, CHr, serum hepcidin and C-Reactive Protein (CRP)]. These determinations were collected one week after the end of iron treatment. In the second week of treatment only hematimetria and CHr were collected without suspension of iron treatment.
The hematimetria and the CHr were measured with a Roche® XE 5000 autoanalyzer. The hepcidin was measured with the DRG® Hepcidin ELISA (EIA-4705 DRG International Inc., USA), a solid phase enzyme-linked immunosorbent assay (this kit is intended for research use only).
Study objectives
In this study we aim to find out if a low dose of iron therapy dosed in small amounts at each HD session is enough as maintenance therapy to replenish the lack or iron in HD patients. Our primary goal was to observe whether the administration of 20 mg of sucrose iron in every HD session for four weeks increases the EA (measured by reticulocyte count) and if the FI improves (measured by CHr). As a secondary objective of the study, we included the behavior of such parameters in patients that have been receiving iron or not as a maintenance treatment prior to the study, its behavior according to the inflammation grade before the study, as well as the safety and tolerability of this IV iron maintenance treatment. Analyses were performed in accordance with the Declaration of Helsinki and the guidelines of the institutional review board of the hospital.
Statistical analysis
Statistics were performed using IBM SPSS Statistics (SPSS, Chicago, IL). Categorical variables were expressed as percentages, continuous variables were expressed as mean ± standard deviation. The Student’s test for multiple comparisons was applied to compare groups, as appropriate. Bivariate linear correlations were expressed by Pearson’s correlation coefficients. A p value <0.05 was considered as statistically significant.
The sample size calculation was based on the assumption that the overall erythropoiesis activity (measured by reticulocyte parameter) and the functional iron (measured by content of CHr) would not change with the administration of 20 mg of sucrose iron in every HD session compared with patients with a higher administration of iron endovenous therapy per week, like a non-inferiority study model.
A difference of >10% in reticulocyte count between patients that received 100 mg/week of iron prior to the study had to be detected to be clinically relevant. With a first-order error of 5% and a power of 95%, a sample size of 45 patients was needed for the study.
Results
Iron administration and erythropoiesis
The reticulocyte levels were not significantly affected along the study (). Likewise, no correlation was observed between the percentage change in reticulocytes and the rest of parameters studied. After 15 days, the Hb, Htc, and RBC levels had significantly descended. However, they returned to levels similar to the basal ones at the end of the study (). We observed a little increase in erythropoietic parameters in patients that had not received iron prior to the study (). On the other hand, we observed a little decrease in such parameters () in patients that received 100 mg of iron a week as a maintenance treatment, with no significant difference in both cases.
Table 1. Summarizes basal parameters at 15 and 30 days of erythropoietic activity in different groups.
Iron administration and iron status evaluation
The CHr levels remained practically unaltered throughout the study (). We observed a little increase in patients that had not received iron prior to the study (). On the other hand, we observed a little decrease of such parameter () in the patients that received a 100 mg of iron a week as a maintenance treatment, with no significant difference in both cases. There was a close correlation between the CHr levels and FI parameters (serum iron and TSAT) at the beginning and the end of the study. This parameter was not correlated in any moment with the serum hepcidin or with the basal CRP ().
Figure 1. The close correlation existing between CHr levels and the commonly used test to evaluate the functional iron (serum iron and TSAT), at the beginning and the end of the study. The CHr levels did not correlate with the serum hepcidin or with the basal CRP. TSAT: Transferrin Saturation; CHr: Content of Hemoglobin Reticulocyte; CRP: C-Reactive Protein.
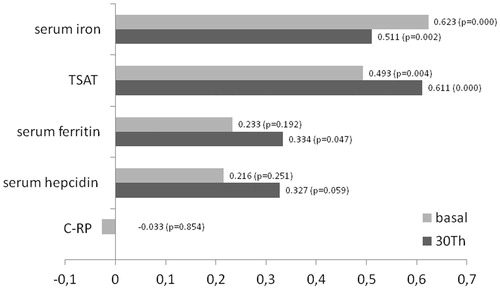
Table 2. Summarizes basal parameters at 15 and 30 days of functional iron and iron status in different groups.
Serum iron values, serum transferrin, TSAT, ferritin, and hepcidin did not significantly change over the study (). We observed an increase in the serum iron levels and TSAT with a decrease in the serum ferritin levels and serum hepcidin () in patients that had not received iron prior to the study. These findings, which did not reach a significant difference, were not observed in patients that were receiving 100 mg of iron a week as a maintenance therapy before the study ().
Inflammation influence
There was not a positive correlation between the CRP levels, EA and FI parameters: CHr (), serum iron (r = −0.144; p = 0.394) or TSAT (r = −0.049; p = 0.772).
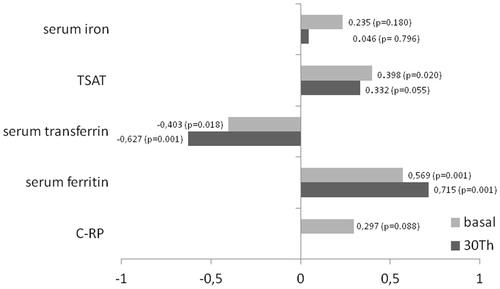
Figure 2. An excellent positive correlation between levels of hepcidin and ferritin, and a similar tendency with CRP. It is possible that patients with high levels of hepcidin also have a higher degree of inflammation and therefore lower levels of transferrin, which entitles higher levels of TSAT. CRP: C-Reactive Protein; TSAT: Transferrin Saturation.
Safety and tolerability
The tolerance of direct iron sucrose administration was good. No serious adverse events were observed beyond the study.
Discussion
The direct administration in the venous line of a small quantity of iron at the end of every HD session does not produce significant changes in the EA. The majority of authors use the Hb or Htc as activity markers. However, these parameters at least need a 60 days observation period,Citation13 the necessary time to replace previously existing erythrocyte for another one generated by the new erythropoietic therapy. We decided to use the reticulocytes count in peripheral blood because of its short half-life (near 24 h),Citation14 and for its rapid presence in the peripheral circulation, allowing its determination at 15 days inclusive.Citation15 By shortening the period of the study, the intercurrent processes as infections, bleeding, or neocytolysis, preventing the outflow of patients from the study were minimized. Other EA markers, like the erythron transferrin uptakeCitation16 or the soluble transferrin receptor,Citation15 also reduce the observation period. However, its determination is more complex and more expensive. An unchanged dose of ESAs throughout the study avoids confounding factors that could be attributed to erythropoiesis effect.
Another primary objective was to evaluate the FI with this therapy. FID is a state in which there is insufficient iron incorporation into erythroid precursors in the face of apparently adequate body iron stores, together with a serum ferritin value within normal limits.Citation17 The classic parameters do not show the sensitivity and specificity required.Citation18,Citation19 The best indicators of FID are the percentage of hypochromic red cells (% HRC) and the CHr, which reflects the recent availability of iron for Hb synthesis.Citation19 CHr >32 pg is indicative of an adequate iron incorporation into the developing erythron.Citation17 In our study, the administration of a low iron bolus dose in every HD session did not change the FI, staying almost invariable in the three measured periods.
Despite of these results, we thought that the iron maintenance therapy used in this study could be indicated in base to the following considerations. In the first place, when we introduce IV iron in a short time, we can avoid a possible denaturation that can happen when iron stays in saline solutions for long time periods. Besides, when iron is administered once patients finish HD session, it prevents any possible dialytic loss. In the second place, these patients have inflammation more frequently, a clinical condition that produce an iron store and availability reduction once administered.Citation17 Therefore, the use in these cases of large, single iron doses can increase the multi-organ iron deposits and do not cover the daily medullary necessity. On the contrary, a dispensation several times a week can increase the iron presence in the bloodstream and the availability in bone marrow. Third, in inflammatory and/or malnutrition states, the transferrin levels decrease, and with it, its capacity of uptake and transport after iron administration. Use of low doses of iron enhance both the uptake and the transport, avoiding the formation of significant amounts of nontranferrin bound iron (NTBI), also called “free iron”, a form of iron that might induce oxidative stress and cellular damage.Citation20
Another important aspect in the iron therapy is the doses that those patients need to balance the annual iron losses. The dose prescribed has been increased in most countries over the past 10–15 years.Citation1,Citation11 However, benefits from IV iron must be balanced against potential risks. Bailie et al. have recently reported a risk increase of 18% of all-cause mortality with a 4 month dose of ≥400 mg/month compared to 100–299 mg/month doses.Citation21 Similarly, Miskulin et al. found a trend of increased risk of infection-related mortality when cumulative iron doses exceeded 1050 mg over 3 months or 2100 mg over 6 months.Citation22 Other authors report similar results.Citation23 We used a dose that can be considered safe (260 mg/month) and besides, we observed that those patients that had been previously receiving maintenance doses larger (400 mg/month), now maintain both EA and FI levels, decreasing the excessive iron exposition and the possible risk of mortality, hospitalization, or both.
The hepcidin has emerged like the main iron regulatory. It is present in all cells involved in iron homeostasis. This peptide act degrading ferroportin, the only known iron export, decreases iron absorption from the gastrointestinal tract and decreases the accessibility of stored iron from macrophages and the hepatocytes.Citation24 Its synthesis is up regulated in the liver by the iron status, and the setting of chronic inflammation is cleared by the kidneys, so its levels rise as the renal disease advances.Citation25 In our study, we found high hepcidin levels similar to reports made by other authors in HD patients.Citation26,Citation27 We also observed also an excellent correlation between the ferritin and hepcidin, and a tendency between the hepcidin and RCP levels (). Both findings are expected because the hepcidin and ferritin share the same mechanism of regulations.Citation25 Nowadays, the repeated IV iron administration would increase blood hepcidin levels and thereby increase the subsequent iron blockade.Citation28 Consistent with continued dysregulation, a high iron concentration was found in the liver of HD patients who received IV iron therapy.Citation18 On the other hand, it has been recently shown that hepcidin levels were associated with fatal and nonfatal cardiovascular events, even after adjustment for inflammation.Citation29 Therefore, that is interesting to us, to observe the behavior of the hepcidin levels in the 13 patients that had not received iron maintenance therapy prior to the study. After the administration of this pattern in these patients, the FI parameters increased slightly, but the increase of hepcidin levels was avoided (just like the ferritin ones).
From the observations of our study, we can conclude that a lower dose of iron maintenance therapy in HD patients administered in small amounts at the end of every HD session is as effective as larger amounts of iron supplementations and allows reducing the iron overload and the adverse effects.
Our study presents some limitations: It is observational and not controlled; besides, the sample is small.
Another possible confusion effect is that not all patients had received the same doses of IV iron maintenance before the study. Prospective studies are necessary, with larger samples and under the same basal conditions that can measure the efficiency of this pattern and its possible effect in the tissular deposits and in the NTBI.
Acknowledgments
The authors acknowledge to Amgen, the adequate Kit for the hepcidin determination.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Charytan DM, Pai AB, Chan CT, et al. Considerations and challenges in defining optimal iron utilization in hemodialysis. J Am Soc Nephrol. 2015;26:1238–1247.
- Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339:584–590.
- Drueke TB, Locatelli F, Clyne N, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355:2071–2084.
- Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–2098.
- Wetmore JB, Peng Y, Monda KL, et al. Trends in anemia management practices in patients receiving hemodialysis and peritoneal dialysis: A retrospective cohort analysis. Am J Nephrol. 2015;41:354–361.
- Rhee CM, Kalantar-Zadeh K. Is iron maintenance therapy better than load and hold? J Am Soc Nephrol. 2013;24:1028–1033.
- Freburger JK, Ellis AR, Kshirsagar AV, Wang L, Brookhart MA. Comparative short-term safety of bolus versus maintenance iron dosing in hemodialysis patients: A replication study. BMC Nephrol. 2014;15:154–163.
- KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease, Kidney Int Suppl. 2012;2:279–335.
- Locatelli F, Bárány P, Covic A, et al. Kidney disease: improving global outcomes guidelines on anaemia management in chronic kidney disease: A European renal best practice position statement. Nephrol Dial Transplant. 2013;28:1346–1359.
- Susantitaphong P, Alqahtani F, Jaber BL. Efficacy and safety of intravenous iron therapy for functional iron deficiency anemia in hemodialysis patients: A meta-analysis. Am J Nephrol. 2014;39:130–141.
- Bailie GR, Larkina M, Goodkin DA, et al. Variation in intravenous iron use internationally and over time: The dialysis outcomes and practice patterns study (DOPPS). Nephrol Dial Transplant. 2013;28:2570–2579.
- Geisser P, Burckhardt S. The pharmacokinetics and pharmacodynamics of iron preparations. Pharmaceutics. 2011;3:12–33.
- Vos FE, Schollum JB, Coulter CV, Doyle TC, Duffull SB, Walker RJ. Red blood cell survival in long-term dialysis patients. Am J Kidney Dis. 2011;58:591–598.
- Krzyzanski W, Brier ME, Creed TM, Gaweda AE. Reticulocyte-based estimation of red blood cell lifespan. Exp Hematol. 2013;41:817–822.
- Lorenzo JD, Rodríguez MM, Martín SS, Romo JM. Assessment of erythropoiesis activity during hemodialysis therapy by soluble transferrin receptor levels and ferrokinetic measurements. Am J Kidney Dis. 2001;37:550–556.
- Cazzola M, Pootrakul P, Huebers HA, Eng M, Eschbach J, Finch CA. Erythroid marrow function in anemic patients. Blood. 1987;69:296–301.
- Thomas DW, Hinchliffe RF, Briggs C, et al. Guideline for the laboratory diagnosis of functional iron deficiency. Br J Haematol. 2013;161:639–648.
- Ferrari P, Dheda S, Betti S, et al. Serum iron markers are inadequate for guiding iron repletion in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:77–83.
- Besarab A, Szczech L. Uses and interpretation of iron studies in patients on chronic dialysis. Semin Dial. 2014;27:579–581.
- Macdougall IC, Geisser P. Use of intravenous iron supplementation in chronic kidney disease: An update. Iran J Kidney Dis. 2013;7:9–22.
- Bailie GR, Larkina M, Goodkin DA, et al. Data from the dialysis outcomes and practice patterns study validate an association between high intravenous iron doses and mortality. Kidney Int. 2015;87:162–168.
- Miskulin DC, Tangri N, Bandeen-Roche K, et al. Intravenous iron exposure and mortality in patients on hemodialysis. Clin J Am Soc Nephrol. 2014;9:1930–1939.
- Fishbane S, Mathew AT, Wanchoo R. Intravenous iron exposure and outcomes in patients on hemodialysis. Clin J Am Soc Nephrol. 2014;9:1837–1839.
- Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood. 2003;102:783–788.
- Tsuchiya K, Nitta K. Hepcidin is a potential regulator of iron status in chronic kidney disease. Ther Apher Dial. 2013;17:1–8.
- Zaritsky J, Young B, Wang HJ, et al. Hepcidin-a potential novel biomarker for iron status in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:1051–1056.
- Ashby DR, Gale DP, Busbridge M, et al. Plasma hepcidin levels are elevated but responsive to erythropoietin therapy in renal disease. Kidney Int. 2009;75:976–981.
- Gaweda AE, Ginzburg YZ, Chait Y, Germain MJ, Aronoff GR, Rachmilewitz E. Iron dosing in kidney disease: Inconsistency of evidence and clinical practice. Nephrol Dial Transplant. 2015;30:187–196.
- van der Weerd NC, Grooteman MP, Bots ML, CONTRAST Investigators, et al. Hepcidin-25 is related to cardiovascular events in chronic haemodialysis patients. Nephrol Dial Transplant. 2013;28:3062–3071.
