Abstract
Background: The aim of the present study was to investigate the risk factors related to hospital mortality due to infection in kidney recipients with ARDS meeting the Berlin definition.
Methods: Univariate and multivariate logistic regression analysis were used to confirm the independent risk factors related to infection-associated mortality.
Results: From January 2001 to August 2014, a total of 94 recipients with acute respiratory dress syndrome (ARDS) caused by pneumonia following kidney transplantation were enrolled in the present study. The most common type of infection was bacterial (52/94; 55.3%), viral (25/94; 26.6%), and polymicrobial (14/94; 14.9%). The most common ARDS was diagnosed within 6 months after transplantation (76/94; 80.9%). There were 39 deaths in these recipients (39/94; 41.5%). Eleven (11.7%) patients had mild, 47 (50.0%) moderate, and 36 (38.3%) severe ARDS; mortality was 27.3, 27.7, and 63.9%, respectively. The independent predictors of infection-related mortality were serum creatinine level >1.5 mg/dL at ARDS onset (OR 3.5 (95%CI 1.2–10.1), p = 0.018) and severe ARDS (OR 3.6 (95%CI 1.4–9.7), p = 0.009) in the multivariate analysis.
Conclusion: Infection-related mortality in kidney transplant patients with ARDS was associated with high serum creatinine level and severe ARDS.
Introduction
Because of immunosuppression, pneumonia frequently occurs after kidney transplantation, primarily in the first 3–6 months following transplantation when immunosuppressive agents are intensely administered. According to various series, occurrence rates of pneumonia ranged from 4.5% to 20%Citation1–15 and pneumonia accounted for 8–70.3% of all infectious complicationsCitation7,Citation16–19 following kidney transplantation.
Pneumonia is a serious infectious complication in kidney transplant recipients and easily leads to acute respiratory dress syndrome (ARDS)Citation20 which has an occurrence rate ranging from 0.2% to 4.3%Citation21,Citation22 and a mortality rate of 22.5–67%.Citation4,Citation21–28 The mortality rate was reported to be nearly 100% in ARDS patients on ventilation.Citation29,Citation30 In the setting of the use of thymosin α1, the death rate was greatly reduced from 50% to 21.9% in kidney recipients with ARDS due to cytomegalovirus infection.Citation31 The most common etiology of ARDS in kidney recipients is sepsis, but it may also be caused by immunosuppressive therapy prescribed for these patients.Citation32,Citation33
The predictors of mortality in kidney recipients with ARDS secondary to pneumonia have not been well studied. We therefore sought to (i) analyze the microbiological spectrum involved in ARDS in kidney transplant recipients and (ii) evaluate the factors associated with hospital mortality owing to infection.
Material and methods
Ethics statement
The study protocol, which included participants providing written consent prior to the study, was approved by the Third Xiangya Hospital of Central South University and the Zhongnan Hospital of Wuhan University, Medical Ethical Committee.
Study population
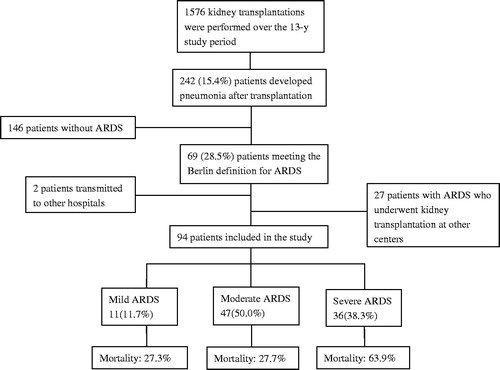
A total of 1576 patients underwent allograft kidney transplantation between January 2001 and August 2014 at the Third Xiangya Hospital of Central South University, Changsha, China, and Zhongnan Hospital of Wuhan University, Wuhan, China. Of them, 67 kidney recipients diagnosed with ARDS were included. Twenty-seven kidney patients with ARDS who underwent transplantation at other centers and were transferred to the two transplant centers involved also were enrolled. Patients with pneumonia without ARDS and patients with ARDS being transferred to other hospitals were excluded.
All the transplanted kidneys were obtained from deceased, living-related donors or donors after cardiac death (DCD). There were no marked X-ray abnormalities in any recipient prior to transplantation. The matched donors and recipients had the same blood type and shared at least two human leukocyte antigen haplotypes (HLA-A, B, DR). The maintenance immunosuppressive medications consisted of a calcineurin inhibitor (tacrolimus or cyclosporine A) combined with mycophenolate mofetil and steroids. Antiviral prophylactic or preemptive therapies directed against cytomegalovirus in all patients. Of these 94 patients enrolled, 12 (12.8%) received cytomegalovirus prophylaxis agent, namely, ganciclovir; five (5.3%) received Pneumocystis carinii prophylaxis agent, namely, sulfamethoxazole. All kidney recipients with ARDS were provided with non-invasive or invasive ventilatory support. Non-invasive and endotracheal mechanical ventilation were instituted at the discretion of the attending physicians. In patients with ARDS, all immunosuppressive agents were withdrawn other than 40 mg of methylprednisolone which was administered 1–4 times per day.
Study design and data collection
A retrospective study was performed to determine the predictors of hospital mortality due to pulmonary infection in kidney transplant recipients. Clinical characteristics including age, sex, type of donors, underlying diseases, times of kidney transplantation, comorbidities, use of antilymphocyte globulin prior to ARDS for induction or rejection treatment, acute rejection episodes and major infection within 3 months prior to ARDS, the body temperature at ARDS onset and the highest body temperature during the ARDS course, time of ARDS onset, type of organisms, nosocomial origin of infection, use of anti- cytomegalovirus and Pneumocystis carinii agents, thymosin α1 and γ-globulin prior to and after ARDS, septic shock, and laboratorial data at ARDS onset were collected. The laboratory variables included serum creatinine levels, serum albumin levels, white blood cell (WBC) count, platelet count, and lymphocyte count. All kidney recipients were with a follow-up period of at least 2 months after the onset of ARDS.
Definitions
ARDS was diagnosed when arterial oxygen partial pressure/oxygen inspiratory fraction (PaO2/FiO2) ratio ≤300, with positive end expiratory pressure ≥5 cm H2O, accompanied with (1) within 1 week of a known clinical insult new-onset or deteriorating respiratory symptoms; (2) opacities in two lungs on chest radiographs; and (3) cardiac dysfunction or fluid overload not fully responsible for respiratory failure. The severity of ARDS was categorized as mild (200 mmHg < PaO2/FiO2 ≤ 300 mmHg); moderate (100 mmHg < PaO2/FiO2 ≤ 200 mmHg), or severe (PaO2/FiO2 ≤ 100 mmHg). Both ARDS and its severity were on the basis of according to the Berlin criteria.Citation34 The cut off value of serum creatinine (>1.5 mg/dL) as a marker of renal dysfunction was basis on previous studies.Citation35,Citation36
Septic shock was diagnosed as hypotension unresponsive to intravenous fluid challenge with the need for vasopressor.Citation16 Infection-related mortality was determined when it related to clinical signs of active infection, and when there was no evidence of any other attributable cause.
Statistical analysis
Continuous data were reported as mean ± standard deviation for normally distributed data, and as median (interquartile range [IQR]) for non-normally distributed data. For continuous variables, Student’s t-test or Mann–Whitney’s U-test was used, as appropriate. Chi-square analysis or Fisher’s exact tests was used for comparisons of categorical data. Multiple forward logistic regression was performed to confirm predictors independently related with infection-associated mortality. Only the variables of p < 0.1 in the univariate analysis were included in the multiple logistic regression model (except for gender which in generally should be included in the multivariable analysis). Odds ratio (OR) (95% confidence interval [CI]) were calculated. A two-sided value of p < 0.05 was assumed to represent statistical significance. SPSS version 17.0 (SPSS Inc., Chicago, IL) was used for the statistical analysis.
Results
A total of 1576 patients underwent kidney transplantation during the study period. Of these patients, 67 kidney recipients diagnosed with ARDS owing to pneumonia after kidney transplantation were enrolled and 27 patients with ARDS who underwent transplantation and were transferred to the two hospitals involved were enrolled, as shown in .
Demographic, laboratorial, and clinical characteristics of patients are shown in . The main causes of kidney failure were chronic glomerulonephritis (62.8%), diabetic nephropathy (9.6%), and polycystic kidney diseases (4.3%). The majority of grafts were obtained from a deceased donor (51.1%). The mean age of patients at ARDS onset was 39.5 (range 16–65) years. Among the 94 enrolled cases, 76 were male and 18 were female. The majority of ARDS cases (90/94; 95.7%) occurred with the first kidney transplantation and only four cases with the second kidney transplantation. A total of 41 (43.6%) patients had at least one acute rejection episode, and 17 (18.1%) at least one major infection within 3 months before ARDS was diagnosed. Of all these 94 patients, HBV infection was in 17.0% and HCV infection in 3.2% cases. Five patients developed new-onset diabetes mellitus between transplantation and the development of ARDS.
Table 1. Demographic, laboratory, and clinical variables of 94 renal transplant patients diagnosed with ARDS.
Around 80% (76/94) of ARDS developed within 6 months after kidney transplantation. Kidney recipients underwent an average duration of 102.5 d between transplantation and ARDS onset (IQR: 82.8–153.3 d). They had a median body temperature of 38.2 °C (IQR: 37.5–39.0 °C) at ARDS onset and the highest temperature of 39.4 °C (IQR: 38.9–39.7 °C) during ARDS course. Eight (8.5%) patients experienced septic shock at ARDS onset.
Eleven (11.7%) and 30 (31.9%) patients received administration of γ-globulin, seven (7.4%) and 44 (46.8%) thymosin α1, five (5.3%) and three (3.2%) anti-Pneumocystis carinii agent sulfamethoxazole, and 31 (33.0%) and 39 (41.5%) anti-viral agent ganciclovir prior to and after ARDS, respectively.
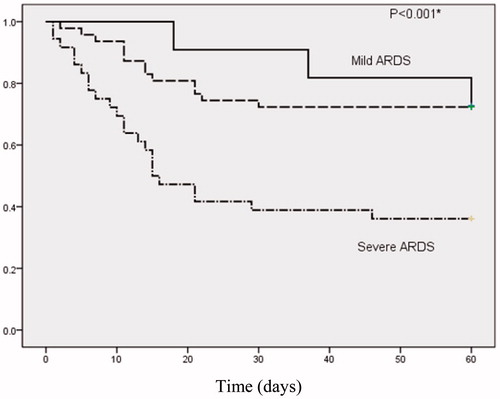
Hospital mortality due to infection was 41.5% (39 of 94 patients) overall. However, the rate sharply increased to 75% (6/8) when ARDS onset accompanied by septic shock. Eleven (11.7%) patients had mild, 47 (50.0%) moderate, and 36 (38.3%) severe ARDS; mortality was 27.3, 27.7, and 63.9%, respectively (p = 0.002, .
Figure 2. Kaplan–Meier curves for two-month survival after ARDS was diagnosed according to severity category in the Berlin definition. The uppermost line indicates mild ARDS, middle line moderate ARDS and downmost line severe ARDS. *p Values denotes the difference in survival among three groups using Log-rank test.
The most common type of infection was bacterial (52/94; 55.3%), viral (25/94; 26.6%), and polymicrobial (14/94; 14.9%). A total of 178 agents were responsible for pneumonia in these 94 kidney recipients enrolled. Most of these agents were Gram-negative bacteria (62.4%), followed by Gram-positive bacteria (15.7%), cytomegalovirus (16.9%), and fungi (6.2%). Acinetobacter baumanii (18.5%), Pseudomonas aeruginosa (11.2%), and Staphylococcus aureus (9.6%) were the most prevalent bacteria. listed the distribution of causative organisms among kidney recipients diagnosed with ARDS.
Table 2. Classification and percentage of organisms in renal recipients with acute respiratory distress syndrome.
showed the univariate analyses of the relationship between ARDS onset within 6 months after operation (p = 0.016), severe ARDS (p = 0.001), septic shock at ARDS onset (p = 0.044), and serum creatinine level >1.5 mg/dL (p = 0.003) and risk for hospital mortality. There was a trend suggesting that platelet count <100,000/mm3 (p = 0.099) and age (p = 0.063) may have a role in infection-related mortality in kidney recipients with ARDS.
Table 3. Univariate analysis: risk factors for hospital death in renal recipients with ARDS.
showed that in the multivariate analysis, the independent predictors of infection-associated mortality were serum creatinine level >1.5 mg/dL at ARDS onset (OR 3.5 (95%CI 1.2–10.1), p = 0.018) and severe ARDS (OR 3.6 (95%CI 1.4–9.7), p = 0.009). No other variable that was associated with hospital mortality in the univariate analysis remained significant in the multivariate analysis.
Table 4. Multivariable analysis: risk factors for infection-related death in renal recipients with ARDS.
Discussion
Infections following kidney transplantation mainly affect the lungs and the airways. Pneumonia easily develops into ARDS which makes kidney recipients face significant morbidity and mortality. The present study showed a high incidence of ARDS (4.4%) and a high rate of infection-related mortality (41.5%) among kidney recipients with ARDS in China. We observed a higher incidence of ARDS in contrast with the previous report from American, conducted by Shorr et al.,Citation22 who noted a low ARDS incidence of 0.2% in kidney recipients, probably as a result of a routine prophylaxis regimen. Our higher incidence may be a result of unhygienic conditions, stronger immunosuppressive agents, and the absence of a routine chemoprophylaxis for cytomegalovirus and Pneumocystis carinii, although all patients underwent a routine antiviral preemptive therapy directed against cytomegalovirus. Our result was in accordance with another previous study from China, which claimed that the incidence rate of ARDS caused by severe pneumonia was 4.3% (21 of 486 kidney recipients).Citation27 Our present study regarding high mortality was extremely similar to two previous studiesCitation21,Citation37 which suggested the mortality rate ranged from 38.1% to 45.5% in kidney recipients with ARDS due to pneumonia.
In our patients, A. baumanii and P. aeruginosa were the leading microbial isolates, in accordance with a previous studyCitation21 regarding kidney recipients with respiratory failure due to bacterial pneumonia. Hoyo et al.Citation4 also revealed that the most commonly isolated microorganism in kidney recipients with nosocomial pneumonia was P. aeruginosa.
We identified increased serum creatinine as a 3.5-fold greater risk factor for hospital mortality, in accordance with the studyCitation21 which reported high serum creatinine level to be a mortality determinant in kidney recipients with respiratory failure due to severe bacterial pneumonia. In a multicentre study, Canet et al.Citation23 also suggested that kidney function was independently associated with dialysis-free survival in kidney recipients with acute respiratory failure. Our previous studyCitation36 claimed that high serum creatinine levels significantly associated with increased mortality in deceased donor liver transplant recipients with bloodstream infections.
We also found severe ARDS was significantly associated with hospital mortality, in line with a recent studyCitation38 suggesting that severe ARDS was a risk factor for higher mortality in patients with malignancies.
In a recent letterCitation39 published in journal “Intensive Care Medicine”, our study group retrospectively analyzed the microbiological spectrum and evaluated the factors associated with infection-related mortality in kidney recipients with ARDS within 6 months after transplantation between 2004 and 2014 and 72 patients were enrolled. We revealed hospital mortality was associated with high serum creatinine level, severe ARDS, and use of tacrolimus. However, comprehensive information regarding the effect of variables on mortality in kidney recipients with ARDS due to pneumonia developing at any time after transplantation is urgently needed. The present manuscript, therefore, studied all 94 kidney recipients with ARDS within and beyond 6 months after transplantation between 2001 and 2014. We found, in the present study, only high serum creatinine level and severe ARDS other than use of tacrolimus were risk factors for hospital mortality. The present study highlighted the importance of both high serum creatinine level and severe ARDS as predictors of mortality among kidney recipients with ARDS within 6 months or at any time after transplantation. The decrease of importance of use of tacrolimus as a predictor of mortality among kidney recipients with ARDS at any time after transplantation was related to a low dosage of tacrolimus needed to be maintained beyond 6 months after transplantation therefore producing a low immunosuppressive effect.
To the best of our knowledge, the prognosis factors in the kidney transplant recipient’s population with ARDS due to pneumonia caused by a variety of micro-organisms have not been investigated previously; we first found high serum creatinine level and severe ARDS to be risk factors for infection-related mortality in these patients. Still, further well-designed studies with larger sample size were warranted.
The present study has some limitations, including the retrospective nature of our data collection which could be affected by various forms of ascertainment and recall bias. The strengths of our study include (i) this was a double-centre study; (ii) we included patients over a 13-year period; and (iii) we had a relatively larger patient sample with ARDS due to pneumonia than the other similar studies.
Conclusions
In summary, the present study confirms that a high rate of morbidity exists for patients with ARDS following kidney transplantation and that although immunosuppressive agents were timely withdrawn at an early stage when the kidney recipients were diagnosed with pneumonia, glucocorticosteroids were regularly used, antimicrobial and antiviral agents were appropriately administrated, and mechanical ventilation therapy was used, ARDS accompanied by high serum creatinine level and severe ARDS are associated with high mortality rates. Therefore, early diagnosis, strategic prevention and active treatment of pneumonia, active nutritional support, and reducing the incidence of ARDS are crucial for lowering the mortality rates among these immunocompromised patients.
Disclosure statement
The authors report that they have no conflicts of interest.
References
- Dizdar OS, Ersoy A, Akalin H. Pneumonia after kidney transplant: Incidence, risk factors, and mortality. Exp Clin Transplant. 2014;12:205–211.
- Luo W. Pulmonary infections in renal transplant recipients [in Chinese]. Zhonghua Yi Xue Za Zhi. 1991;71:246–248.
- Jha R, Narayan G, Jaleel MA, et al. Pulmonary infections after kidney transplantation. J Assoc Physicians India. 1999;47:779–783.
- Hoyo I, Linares L, Cervera C, et al. Epidemiology of pneumonia in kidney transplantation. Transplant Proc. 2010;42:2938–2940.
- Pourmand G, Salem S, Mehrsai A, et al. Infectious complications after kidney transplantation: A single-center experience. Transpl Infect Dis. 2007;9:302–309.
- Garcia-Prado ME, Cordero E, Cabello V, et al. Infectious complications in 159 consecutive kidney transplant recipients. Enferm Infecc Microbiol Clin. 2009;27:22–27.
- Veroux M, Giuffrida G, Corona D, et al. Infective complications in renal allograft recipients: Epidemiology and outcome. Transplant Proc. 2008;40:1873–1876.
- Fishman JA, Rubin RH. Infection in organ-transplant recipients. N Engl J Med. 1998;338:1741–1751.
- Charfeddine K, Zaghden S, Kharrat M, et al. Infectious complications in kidney transplant recipients: A single-center experience. Transplant Proc. 2005;37:2823–2825.
- Kalra V, Agarwal SK, Khilnani GC, et al. Spectrum of pulmonary infections in renal transplant recipients in the tropics: A single center study. Int Urol Nephrol. 2005;37:551–559.
- Bowie DM, Marrie TJ, Janigan DT, et al. Pneumonia in renal transplant patients. Can Med Assoc J. 1983;128:1411–1414.
- Huertas VE, Port FK, Rozas VV, et al. Pneumonia in recipients of renal allografts. Arch Surg. 1976;111:162–166.
- Ettinger NA, Trulock EP. Pulmonary considerations of organ transplantation. Part I. Am Rev Respir Dis. 1991;143:1386–1405.
- Edelstein CL, Jacobs JC, Moosa MR. Pulmonary complications in 110 consecutive renal transplant recipients. S Afr Med J. 1995;85:160–163.
- Sileri P, Pursell KJ, Coady NT, et al. A standardized protocol for the treatment of severe pneumonia in kidney transplant recipients. Clin Transplant. 2002;16:450–454.
- Carvalho MA, Freitas FG, SilvaJunior HT, et al. Mortality predictors in renal transplant recipients with severe sepsis and septic shock. PLoS One. 2014;9:e111610.
- Bige N, Zafrani L, Lambert J, et al. Severe infections requiring intensive care unit admission in kidney transplant recipients: Impact on graft outcome. Transpl Infect Dis. 2014;16:588–596.
- Alangaden GJ, Thyagarajan R, Gruber SA, et al. Infectious complications after kidney transplantation: Current epidemiology and associated risk factors. Clin Transplant. 2006;20:401–409.
- Mouloudi E, Massa E, Georgiadou E, et al. Infections related to renal transplantation requiring intensive care admission: A 20-year study. Transplant Proc. 2012;44:2721–2723.
- Dong B, Wang Y, Wang G, et al. A retrospective study of cytomegalovirus pneumonia in renal transplant patients. Exp Ther Med. 2014;7:1111–1115.
- Shih CJ, Tarng DC, Yang WC, et al. Immunosuppressant dose reduction and long-term rejection risk in renal transplant recipients with severe bacterial pneumonia. Singapore Med J. 2014;55:372–377.
- Shorr AF, Abbott KC, Agadoa LY. Acute respiratory distress syndrome after kidney transplantation: Epidemiology, risk factors, and outcomes. Crit Care Med. 2003;31:1325–1330.
- Canet E, Osman D, Lambert J, et al. Acute respiratory failure in kidney transplant recipients: A multicenter study. Crit Care. 2011;15:R91.
- Washer GF, Schroter GP, Starzl TE, et al. Causes of death after kidney transplantation. JAMA. 1983;250:49–54.
- Candan S, Pirat A, Varol G, et al. Respiratory problems in renal transplant recipients admitted to intensive care during long-term follow-up. Transplant Proc. 2006;38:1354–1356.
- Kirilov D, Cohen J, Shapiro M, et al. The course and outcome of renal transplant recipients admitted to a general intensive care unit. Transplant Proc. 2003;35:606.
- Sun Q, Liu Z, Chen J, et al. An aggressive systematic strategy for acute respiratory distress syndrome caused by severe pneumonia after renal transplantation. Transpl Int. 2006;19:110–116.
- Tu GW, Ju MJ, Zheng YJ, et al. An interdisciplinary approach for renal transplant recipients with severe pneumonia: A single ICU experience. Intensive Care Med. 2014;40:914–915.
- Von Willebrand E, Pettersson E, Ahoner J, et al. CMV infection, class II antigen expression and human kidney allograft rejection. Transplantation. 1986;42:364–367.
- Capulong MG, Mendoza M, Chavez J. Cytomegalovirus pneumonia in renal transplant patients. Transplant Proc. 1998;30:3151–3153.
- Ji SM, Li LS, Sun QQ, et al. Immunoregulation of thymosin alpha 1 treatment of cytomegalovirus infection accompanied with acute respiratory distress syndrome after renal transplantation. Transplant Proc. 2007;39:115–119.
- Walton GD, Gualtieri RJ, Ala H. Antithymocyte globulin induced adult respiratory distress syndrome. Arch Intern Med. 1998;158:1380.
- Dean NC, Amend WC, Matthay MA. Adult respiratory distress syndrome related to antilymphocyte globulin therapy. Chest. 1987;91:619–620.
- Ranieri VM, Rubenfeld GD, ARDS Definition Task Force, et al. Acute respiratory distress syndrome: The Berlin Definition. JAMA. 2012;307:2526–2533.
- Shi SH, Kong HS, Xu J, et al. Multidrug resistant gram-negative bacilli as predominant bacteremic pathogens in liver transplant recipients. Transpl Infect Dis. 2009;11:405–412.
- Wan QQ, Ye QF, Ming YZ, et al. The risk factors for mortality in deceased donor liver transplant recipients with bloodstream infections. Transplant Proc. 2013;45:305–307.
- Sun Q, Li L, Ji S, et al. Variation of CD4+ and CD8+ T lymphocytes as predictor of outcome in renal allograft recipients who developed acute respiratory distress syndrome caused by cytomegalovirus pneumonia. Transplant Proc. 2005;37:2118–2121.
- Azoulay E, Lemiale V, Mokart D, et al. Acute respiratory distress syndrome in patients with malignancies. Intensive Care Med. 2014;40:1106–1114.
- Wan Q, Zhang P, Ye Q. Acute respiratory distress syndrome in kidney transplant recipients. Intensive Care Med. 2015;41:373–374.