Abstract
Important safety concerns have recently emerged regarding the use of sodium polystyrene sulfonate (Kayexalate), a cation-exchange resin commonly used for the treatment of hyperkalemia. We implemented an electronic alert system at a tertiary care academic medical center to warn providers of the safety concerns of Kayexalate. We assessed the number of Kayexalate prescriptions per month, as well as the number of grams of Kayexalate ordered per month, one year before versus one year after implementing the alert. The mean (±SD) number of Kayexalate orders decreased from 123 (±12) to 76 (±14) orders/month (38% absolute reduction, p < 0.001) after implementing the alert. Additionally, the mean (±SD) amount of Kayexalate prescribed decreased from 3332 (±329) to 1885 (±358) g/month (43% absolute reduction, p < 0.001). We conclude that an electronic alert is an effective tool to decrease Kayexalate ordering.
Introduction
Sodium polystyrene sulfonate (Kayexalate) is a cation-exchange resin commonly used for the treatment of hyperkalemia. However, surprisingly little evidence supports its efficacy,Citation1 since Kayexalate is a “grandfathered” drug that was approved by the Food and Drug Administration prior to passage of the Kefauver Harris Amendment of 1962. Moreover, important safety concerns have recently emerged: a meta-analysis identified 58 cases of serious adverse events, with colonic necrosis being the most common and resulting in a 33% mortality rate among affected patients.Citation2 Electronic alerts have the potential to alter provider ordering of medications,Citation3,Citation4 but have not been applied to Kayexalate. We therefore implemented an electronic alert system to determine its’ effectiveness in decreasing ordering of Kayexalate.
Methods
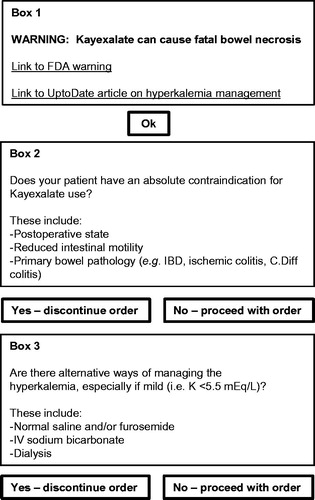
This project was undertaken as a quality improvement initiative at Massachusetts General Hospital (Boston, MA), and as such the requirement for institutional review board supervision was waived. On November 1, 2012, we introduced an electronic alert that was triggered by all attempted Kayexalate orders (). Following an attempted Kayexalate order by a prescriber, the electronic alert appeared immediately on the computer screen and required the prescriber to select the appropriate boxes before proceeding (). If Kayexalate ordering was attempted more than once for the same patient, a separate alert appeared each time. The standardized Kayexalate prescription at Massachusetts General Hospital is a 60 mL solution containing 15 g of sodium polystyrene sulfate (Carolina Medical Products, Farmville, NC). Each 60 mL dose contains 21.5 mL of sorbitol.
We compared the number of Kayexalate prescriptions per month, as well as the number of grams of Kayexalate ordered per month, one year before versus one year after implementing the alert using univariate linear regression. To account for fluctuations in rates of hyperkalemia over time, we normalized the number of Kayexalate orders per month and the number of grams of Kayexalate ordered per month relative to every 1,000 hyperkalemia cases per month, defined by our hospital laboratory as serum potassium ≥4.9 mEq/L. To account for fluctuations in rates of Kayexalate ordering over time that may have occurred independently of our intervention, we used generalized linear mixed models to adjust for the effect of time on the number and amount of Kayexalate orders during the two-year study period. Using an identical approach, we also performed a subgroup analysis to evaluate the effect of the alert on Kayexalate ordering among patients with mild hyperkalemia, which we defined as serum potassium 4.9–5.4 mEq/L.
Results
Comparing the year prior to the alert versus the year after the alert, the mean (±SD) number of Kayexalate orders decreased from 123 (±12) to 76 (±14) orders/month (38% absolute reduction, p < 0.001) (). Additionally, the mean (±SD) amount of Kayexalate prescribed decreased from 3332 (±329) to 1885 (±358) g/month (43% absolute reduction, p < 0.001) (). After adjusting for the longitudinal trend in Kayexalate ordering, the effect of the alert on the number and amount of Kayexalate orders was attenuated, but remained significant with respect to the amount of Kayexalate prescribed (p = 0.004) ().
Figure 2. Kayexalate ordering before and after implementation of the electronic alert (A) Number of Kayexalate orders/month, normalized to 1,000 hyperkalemia values. (B) Amount of Kayexalate ordered/month (grams), normalized to 1,000 hyperkalemia values.
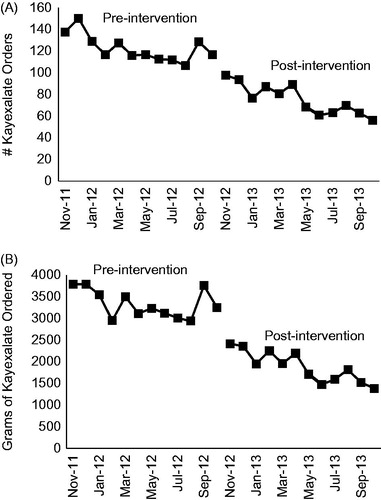
Table 1. Effect of an electronic alert on Kayexalate ordering. The multivariate model is adjusted for trends in Kayexalate ordering over time.
The proportion of all Kayexalate orders administered for mild hyperkalemia, which we defined as serum potassium 4.9–5.4 mEq/L, decreased from 52% before the alert to 47% after the alert (χ2 test, p = 0.001). The mean (±SD) number of Kayexalate orders for mild hyperkalemia decreased from 80 (±10) to 44 (±13) orders/month (45% absolute reduction, p < 0.001), and the mean (±SD) amount of Kayexalate prescribed decreased from 2038 (±261) to 1049 (±324) g/month (49% absolute reduction, p < 0.001). After adjusting for the longitudinal trend in Kayexalate ordering, the effect of the alert on the number and amount of Kayexalate orders was attenuated, but remained significant with respect to the amount of Kayexalate prescribed (p = 0.03) (), similar to the results obtained in the overall analysis.
Table 2. Effect of an electronic alert on Kayexalate ordering – limited to patients with mild hyperkalemia. Mild hyperkalemia was defined as serum potassium 4.9 to 5.4 mEq/L. The multivariate model is adjusted for trends in Kayexalate ordering over time.
Discussion
We conclude that an electronic alert was an effective tool to decrease Kayexalate ordering. However, despite our alert a large proportion (47%) of Kayexalate orders continued to be administered for mild elevations in serum potassium. Thus, further targeted educational interventions are needed to decrease Kayexalate ordering in patients with mild hyperkalemia, many of whom can likely be treated effectively with alternative therapies.
Novel cation exchangers such as Patiromer and Sodium Zirconium CyclosilicateCitation5,Citation6 appear to be safe and effective alternatives to Kayexalate, though large postmarketing surveillance studies will be needed to definitively establish their safety, particularly for rare but serious adverse events. Interestingly, the wholesale cost of a 30-day supply of Patiromer ($595) appears to be comparable to that of Kayexalate ($522). Thus, if postmarketing surveillance studies confirm initial observations of similar efficacy and an enhanced safety profile compared to Kayexalate, it is likely that these novel agents will be increasingly used in the treatment of hyperkalemia.
We acknowledge several limitations, including single center design and lack of clinical information. Specifically, we were unable to collect patient- or provider-specific data, which might have provided insight into how providers interacted with the alert system, including reasons providers may have chosen to order Kayexalate despite the alert. We note an apparent temporal trend in decreased Kayexalate ordering over the course of our study period, which may have occurred independently of our intervention, perhaps as a result of increasing awareness of Kayexalate-related adverse events. A strength of our analysis is that we adjusted for this trend in our generalized linear mixed models, and still showed a significant effect of our intervention on the amount of Kayexalate ordered.
Our study highlights the potential for an electronic alert to decrease Kayexalate ordering. Institutions seeking to decrease Kayexalate ordering, particularly when there is an absolute contraindication or in cases of only mild hyperkalemia, should consider implementing a similar alert system. Future studies should test whether additional targeted interventions using clinical decision support systems can be effective in decreasing Kayexalate ordering.
Disclosure statement
G.D.S. reports having received a contract from MetaWare to study decision-support software. D.B.M. reports having consulted for ZS Pharma and for Up-to-date.
Funding
D.E.L. is supported by K23DK106448 from the National Institutes of Health-National Institute of Diabetes and Digestive and Kidney Diseases.
References
- Sterns RH, Rojas M, Bernstein P, Chennupati S. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol. 2010;21:733–735.
- Harel Z, Harel S, Shah PS, Wald R, Perl J, Bell CM. Gastrointestinal adverse events with sodium polystyrene sulfonate (Kayexalate) use: A systematic review. Am J Med. 2013;126:264. e269-224.
- Chin HL, Wallace P. Embedding guidelines into direct physician order entry: simple methods, powerful results. Proc AMIA Symp. 1999;221–225.
- Freedberg DE, Salmasian H, Abrams JA, Green RA. Orders for intravenous proton pump inhibitors after implementation of an electronic alert. JAMA Intern Med. 2015;175:452–454.
- Packham DK, Rasmussen HS, Lavin PT, et al. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med. 2015;372:222–231.
- Weir MR, Bakris GL, Bushinsky DA, et al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015;372:211–221.