Abstract
Objective: Depression and anxiety are prevalent affective disorders in peritoneal dialysis (PD) patients. Recent research has proposed a potential role of apelinergic system in pathogenesis of depression. The present study aimed to evaluate the frequency of depression and anxiety and their potential relation with serum apelin levels among PD patients.
Methods: A total of 40 PD patients were enrolled into the study. Depressive symptoms and anxiety were assessed with the Beck’s Depression Inventory and the Beck’s Anxiety Inventory. Serum apelin-12 levels were measured by immunoenzymatic assays using commercially available ELISA kit for standard human apelin.
Results: Of the patients, 16 (40%) had depression, 20 (50%) had anxiety. The patients with depression and anxiety had a significantly longer time on dialysis (p < 0.001 for both), significantly higher serum apelin (p < 0.001 for both) and C-reactive protein levels (p < 0.001 for both) than those without depression and anxiety. In multivariate analysis, serum apelin was the only parameter associated independently with depression and anxiety scores.
Conclusions: A substantial number of PD patients had depression and anxiety. Increased levels of serum apelin may constitute a significant independent predictor of development of depression and anxiety in PD patients.
Introduction
Depression and anxiety are prevalent affective disorders in peritoneal dialysis (PD) patients.Citation1 Studies have shown that patients with affective disorders are less likely to adhere to their medications.Citation2,Citation3 Since PD patients perform their own dialysis, early diagnosis of depression and anxiety plays an important part in their adherence to the treatment. Nonadherence to the treatment may cause complications such as peritonitis and result in mortality.Citation4,Citation5
Apelin is a recently discovered bioactive peptide which is highly concentrated in the brain. It appears to mediate its effects via a single G protein-coupled receptor subtype, the apelin receptor (APJ).Citation6 Apelin and APJ mRNA expression have been distributed in various tissues, including the brain, gastrointestinal tract, adipose tissue, lung, kidney, liver, and the cardiovascular system.Citation7 To date, 46 different apelin peptides ranging from apelin 55 (proapelin) to apelin 12 have been identified.Citation8 The different forms of apelin and its receptor APJ are called apelinergic system which plays pivotal roles in the regulation of cardiovascular function, angiogenesis, fluid homeostasis, energy metabolism and stress response.Citation9
The apelinergic system has a widespread but selective expression in the CNS. APJ mRNA expression and apelin-immunoreactivity are present in hypothalamic paraventricular nucleus and supraoptic nucleus as well as in extrahypothalamic structures, in particular the cerebroventricular system, lower brainstem structures and also in the anterior lobe of the pituitary where both receptor and ligand are expressed in corticotrophs.Citation6 On the other hand, detection of apelin receptor mRNA in amygdala, hypothalamus, Ammon's horn and dentate gyrus, the components of the limbic system which are involved in coordinated responses to stress and integrate many behavioral reactions particularly to stress and anxiety, has led to the suggestion that apelin may have a role in regulation of emotional behavior.Citation7,Citation10,Citation11,Citation12 Indeed, centrally administered apelin-13 elicited depression-like behavior in mice, which was mediated via APJ receptor.Citation12 However, to our knowledge, there have been no published papers about the effect of apelinergic system on emotion-related behavior on human. In this study, we evaluated the frequency of depression and anxiety and their potential relation with serum apelin levels among PD patients.
Patients and methods
Study population
All PD patients (between 18 and 65 years of age, n = 53) presenting to our outpatient nephrology clinic for follow-up treatment were invited to the study. All the patients had been on PD for more than 6 months (mean PD duration 76.50 (46.50–92.75) months). Patients with malignancy (n = 1), liver disease (n = 1), acute coronary events (n = 1), autoimmune disease (n = 1), dialysis related peritonitis (n = 2) and other infections (n = 2) were excluded in order to avoid possible effects of these comorbid conditions on mental status. Patients who were unwilling to participate in the study (n = 2) and the ones already taking psychiatric medications (n = 3) were also excluded. Ultimately, a total of 40 PD patients (24 males and 16 females; mean age 46.95 ± 11.78 years) were analyzed for the study. Chronic renal failure was attributed to hypertension in eight cases, diabetic nephropathy in four cases, glomerulonephritis in three cases, polycystic kidney disease in two cases, urolithiasis in three cases, amyloidosis in one case, tubulointerstitial nephritis in one case and was undetermined in 18 cases. All patients were on continuous ambulatory PD (CAPD) with four manual exchanges daily using 1500–2500 mL solutions. Glucose concentrations of the solutions were adjusted depending on the patients’ body fluid status. When indicated, the patients received icodextrin or nutritional treatment. The dose of dialysis was prescribed to reach a weekly Kt/V urea target of >1.7. All subjects gave written informed consent, and the study protocol was reviewed and approved by the local ethics committee. The study was in adherence with the principles of the Declaration of Helsinki.
Data collection
Age, gender, marital status, education and work status, comorbidities, dialysis duration, weight and height of the patients were noted down based on patients’ charts. Body mass index (BMI) was calculated by weight in kg/height in m2. Blood pressure (BP) was measured three times in the sitting position, using a manual sphygmomanometer, after the subjects had a rest for 15 min, and the mean of the three readings was used for analysis. The mean arterial pressure (MAP) was calculated using the following equation: MAP = diastolic BP +1/3 × (systolic BP − diastolic BP). Each patient was assessed with Beck’s Anxiety Inventory and the Beck’s Depression Inventory. In order to analyze the relationship of depression and anxiety with serum apelin, the patients were divided into tertiles according to serum apelin levels: low (apelin <0.43 ng/mL), medium (apelin 0.43–0.64 ng/mL), and high (apelin >0.64 ng/mL). A specialist from the psychiatry department was requested to diagnose and treat the patients based on depression and anxiety scores.
Beck’s Depression Inventory
Beck’s Depression Inventory (BDI) is a self-report-inventory used to measure the severity of depression. The questionnaire includes 21 multiple choice items that describe specific depressive symptoms over the past week. Each item is scored on a scale from 0 to 3 and the sum of these gives the total BDI score. Patients having <10 total BDI score are accepted as normal. The total BDI score ≥10 indicates symptomatic patients; 10–18 indicate mild depression, 19–29 indicate moderate depression and ≥30 indicate severe depression.Citation13
Beck’s Anxiety Inventory
Beck’s Anxiety Inventory (BAI) is a self-report-inventory developed by Beck et al. to determine the frequency of anxiety symptoms. It is composed of 21 items. Each item is scored on a scale from 0 to 3 and the sum of these yields the total BAI score. Scores of 0–7 were accepted as negative symptomatology for anxiety. Scores of 8–15 show mild, scores of 16–25 show moderate and scores of 26–63 show severe anxiety.Citation14
Laboratory measurements
Blood samples were obtained by trained personnel from all participants via antecubital venipuncture after a 12-h overnight fast. Complete blood count, glucose, serum albumin, creatinine, ferritin, parathormone (PTH), triglycerides, high density lipoprotein (HDL) cholesterol, low density lipoprotein (LDL) cholesterol, calcium, phosphate, uric acid and high sensitive C-reactive protein (hsCRP) levels were assessed in all participants using standard laboratory techniques for hospital use.
Measurement of serum apelin levels
Blood samples were taken from a peripheral vein after an overnight fast. After standing at room temperature for about 1 h, the samples are centrifuged at 2000 rpm for 10 min and serum was separated. It was immediately frozen at −80 °C until analyzed. Serum apelin-12 levels were measured by immunoenzymatic assays using commercially available ELISA kit for standard human apelin (Phoenix Pharmaceuticals, Burlingame, CA).The levels of apelin are presented as ng/mL.
Statistical analysis
Statistical analysis was performed by using Statistical Package for the Social Sciences software (SPSS, version 18, Chicago, IL). The distribution of the variables was checked by using the Kolmogorov–Smirnov test and histograms. Continuous variables were presented as mean ± standard deviation for the ones with normal distribution and medians and interquartile ranges: Q1–Q3 for the ones with abnormal distribution. Categorical variables were presented as percentages and numbers. Student’s t-test or Mann–Whitney U-test was used to compare the continuous variables between the two groups, depending on their distribution. Categorical variables were compared with chi-square test. Pearson correlation analysis was used to evaluate the relationship between variables. Two different multivariable linear regression analyses were created in order to determine the independent predictors for depression and anxiety scores. Dialysis vintage, CRP and apelin levels were included into both models depending on the results of univariate comparisons. Both models were also adjusted for age and gender. The number and percentages of patients with depression and anxiety were detected and presented as figures, when the x axis represents the tertiles of apelin concentrations. Statistical significance was considered at a two-tailed value of p < 0.05.
Results
Analysis of the data obtained from BDI showed that 16 patients (40%) had total scores ≥10 and were rated as symptomatic for depression. Seven of 16 patients had mild, four had moderate and five had severe depression. Analysis of the BAI scores showed that 20 patients (50%) had anxiety. Eleven of 20 patients had mild, four had moderate and five had severe anxiety. When the patients with depression were compared with those without depression, time on PD was significantly longer in depressive group (p < 0.001). The patients with depression had significantly higher hsCRP levels than non-depressive group (p < 0.001). Serum apelin was significantly higher in depressive patients (p < 0.001). Other demographic and clinical parameters were similar between the two groups (). In patients with anxiety, time on PD was significantly longer than those without anxiety (p < 0.001). Also, the patients with anxiety had a significantly higher serum apelin and hsCRP levels (p < 0.001 for both). Other demographic and clinical parameters were similar between the two groups ().
Table 1. Demographic and clinical parameters in depressive and non-depressive PD patients.
Table 2. Demographic and clinical parameters in PD patients with and without anxiety.
Since univariate analysis revealed between-group differences with regard to dialysis vintage, hsCRP and apelin level both for depression and anxiety scores, we performed a multivariate linear regression analysis including predictive parameters (dialysis vintage, CRP and apelin level). Serum apelin was the only parameter associated independently with depression and anxiety. After adjustment for age and gender, serum apelin still remained significantly associated with depression and anxiety scores (). In Pearson correlation analysis, depression and anxiety scores had strong correlation with serum apelin levels (r = 0.87, p < 0.001; r = 0.89, p < 0.001 respectively, ). The percentage of patients with depression and anxiety increased in a graded fashion from the low- to high-apelin group. The percentage of depressive patients was 7% (one of 14 patients) in low group, 23% (three of 13 patients) in medium group and 92% (12 of 13 patients) in high group. The percentage of patients with anxiety was 7% (one of 14 patients) in low group and 46% (six of 13 patients) in medium group. Interestingly, all patients in the high group (all of 13 patients), who had serum apelin levels >0.64 ng/mL, had anxiety ().
Figure 1. Correlation of serum apelin levels with Beck’s Depression Inventory score (a) and Beck’s Anxiety Inventory score (b).
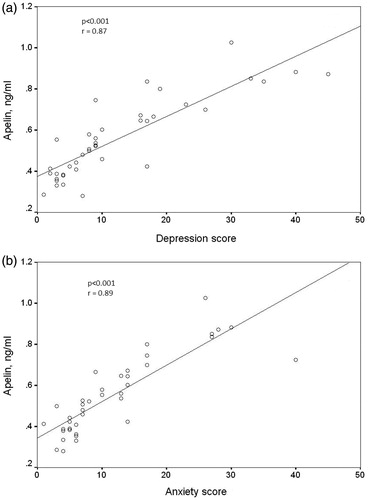
Figure 2. Number of patients with depression (a) and anxiety (b) in apelin tertile groups. The number of patients with depression and anxiety significantly increased with the increasing levels of serum apelin (p < 0.001 for patients with both depression and anxiety).
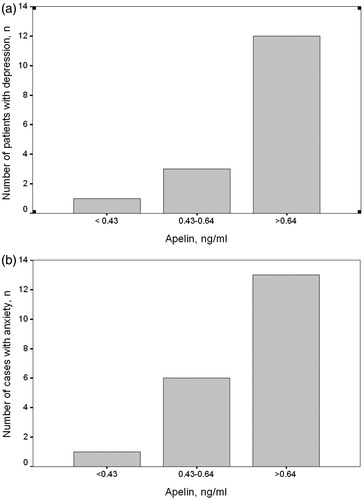
Table 3. Multivariable linear regression analysis showing independent variables associated with depression score (a) and anxiety score (b) as the dependent variables in PD patients.
Discussion
The present study showed that 40% of the PD patients had depression depending on the results of self-reported BDI. Screening for depression in dialysis patients and its prevalence depends on type and cutoff score of diagnostic tool chosen for screening. Using BDI as a diagnostic tool with different cutoff scores ranging from 11 to 17, the frequency of depression was reported between 26% and 62.5%.Citation15–17 Using different depression screening measures, depression prevalence ranging from 14.8% to 52% has also been reported among PD patients.Citation13,Citation18 Eventually, the prevalence of depression in PD patients obtained in the present study is within the suggested range in previous studies.
The frequency of anxiety in PD patients was 50% in the present study. This was a relatively higher rate compared to the other studies which reported distinctive values for anxiety ranging from 11.1 to 30% in PD patients.Citation13,Citation14 The types of diagnostic tool, the special characteristics of the study populations (socio-economic situations, extremely low deceased donor rates and subsequently the longer duration of dialysis treatments in our country) could have affected this result.
In the present study, depression and anxiety scores were found to be closely correlated with dialysis duration. However, researches analyzing the effect of dialysis duration on depression and anxiety provided conflicting results. Furthermore, the population analyzed in these studies is mainly composed of both HD and PD patients or HD patients only. In a study by Martínez-Sanchis et al., dialysis vintage positively correlated with depression scores in HD and PD patients.Citation19 In contrary, another study revealed an inverse correlation between depression and anxiety levels and patients' total length of time on dialysis.Citation20 In a group of HD patients, Pop-Jordanova et al. found a significant negative correlation between depression and duration of dialysis.Citation21 On the other hand, Bossola et al. reported that, symptoms of depression worsened in a meaningful proportion of HD patients over time, whereas, anxiety score decreased or remained stable.Citation22 Finally, Cukor et al. found no relation of length of time on dialysis with depression or anxiety in HD patients.Citation23 The close relation of dialysis duration with depression and anxiety scores in the present study may be partly due to the extremely low deceased donor rates and subsequently the longer duration of dialysis treatments in our country as mentioned above. Additionally, the patient population we analyzed consisted of only the PD patients which may reflect the potential variation in depression and anxiety results obtained with different samples.
In the present study, depression and anxiety scores were found to be closely correlated with hsCRP levels. Several population-based studies have indicated that major depression is associated with inflammatory status possibly due to the proinflammatory cytokine-induced mediators which results in serotonergic deficiencyCitation24–26 Since chronic renal failure is a well-known state of chronic inflammation, relation between CRP and depression scores is not an unexpected finding. However, studies that evaluated possible association between serum CRP levels and depression in PD patients showed mixed results. In consistent with our results, Kalender et al. reported a positive correlation between BDI score and serum CRP in hemodialysis and CAPD patients.Citation27 Also, Ko et al. found that hsCRP significantly affected BDI score in CAPD patients, suggesting a strong association between inflammation and depression.Citation28 In another study using The Hamilton Depression Scale (HAMD), serum CRP closely correlated with depression score in CAPD patients.Citation29 However, other studies could not demonstrate such a relationship between CRP and inflammation.Citation30–32 Using different cutoff points for screening and diagnosis of depression symptoms by assessment tools may be a possible reason for the contradictory results of different studies. On the other hand, the relation between inflammation and anxiety in dialysis patients has not been extensively evaluated. In a study by Preljevic et al., dialysis patients with a CRP level ≥6 mmol/L were found to have four times higher odds of having depressive and/or anxiety disorder.Citation33 Also, Dogan et al. reported a significant correlation between Hamilton Anxiety Scale (HAMA) scores and CRP.Citation34 Although these findings are in concordance with our results, further studies are needed to clarify the potential relation between inflammation and anxiety specifically for PD patients.
Apelin is a more recently discovered adipocytokine, released from white fat tissue and expressed in many tissues including heart, brain, kidneys and endothelium. It has different functions depending on locations of receptors in the organism. Receptors expressed from the vascular system contribute to regulation of blood pressure and stimulate angiogenesis. Apelin is one of most potent stimulants of cardiac contractility.Citation35 Apelin exerts its most important effect on the cardiovascular system, the central nervous system and the renin–angiotensin–aldosterone system.Citation36 Apelin released from the central nervous system has an effect on vasopressin, and neighboring tissues and kidneys through other mediators. Apelin receptors, found in the paraventricular and supraoptic nuclei, exert their effects by sending out signals. In an experimental study, water intake was observed to be affected within 30 min of intraperitoneal apelin administrations to ovolemic rats.Citation7 In another study, following intra-cerebrovascular apelin-17 injections to rats, neurons releasing vasopressin in the hypothalamus were inhibited and dieresis was achieved.Citation37 Relevant studies conducted so far have shown that apelin plays a role in regulation of blood pressures, cardiac contractility, fluid balance, anterior pituitary functions, angiogenesis and inhibition of apoptosis.Citation38,Citation39
Due to abovementioned effects of the apelinergic system, it can be assumed that apelin affects volume regulation, blood pressure and the cardiovascular system in PD patients. However, this is the first study to investigate the relation between the apelinergic system and affective disorders in PD patients.
A remarkable finding of the present study was the strong correlation of serum apelin levels with depression and anxiety scores. This finding was confirmed by the results of multivariate analysis showing that apelin was the only independent predictor for depression and anxiety in PD patients. No studies have evaluated the potential relation between affective disorders and apelin levels in human to date. Only in an experimental study, Lv et al. analyzed the relation between apelin and emotion-related behavior and observed depression-like conditions after apelin-13 was given to the mouse central nervous system.Citation12 Although a direct pathogenetic link cannot be established between apelin and affective disorders, opioid system and inflammation are two factors proposed to be at least partially involved in such association. Recent data has shown that human APJ formed a heterodimer with κ-opioid receptor.Citation40 Since κ-opioid receptor agonists can produce depression or depression-like behaviors in humans and rodents, apelinergic system may be involved in pathogenetic mechanisms of depression through the mediation of opioid system.Citation41,Citation42 Indeed, Lv et al. demonstrated that depression-like behavior induced by apelin-13 was also mediated by κ-opioid receptor.Citation12 On the other hand, an increasing amount of evidence has suggested a close relation between apelin, oxidative stress and inflammation.Citation43–45 Therefore, considering that major depression is associated with inflammatory status, we speculate that apelinergic system may be involved in depressive disorders through inflammatory pathway.
In conclusion, the present study demonstrated that a substantial number of PD patients had depression and anxiety which were associated with serum apelin levels. Therefore, increased levels of serum apelin may constitute a significant independent predictor of development of affective disorders in this particular group of patients. The most important limitation of this study is that it was performed in a single center and included a small sample. Multi-center randomized controlled studies with larger samples are needed to show the relation between the apelinergic system and affective disorders in PD patients.
Ethical approval
All subjects gave written informed consent, and the study protocol was reviewed and approved by the local ethics committee.
Disclosure statement
The authors declare that they have no conflicts of interest related to the contents of this article.
The authors declare that this manuscript has not been published previously and is not currently being assessed for publication by any journal other than “Renal Failure”.
Each author has contributed substantially to the research, preparation and production of the paper and approves of its submission to the Journal.
References
- Park HC, Lee H, Lee JP, et al. Lower residual renal function is a risk factor for depression and impaired health-related quality of life in Korean peritoneal dialysis patients. J Korean Med Sci. 2012;27:64–71.
- DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: Meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–2107.
- Penkower L, Dew MA, Ellis D, Sereika SM, Kitutu JM, Shapiro R. Psychological distress and adherence to the medical regimen among adolescent renal transplant recipients. Am J Transplant. 2003;3:1418–1425.
- Troidle L, Wuerth D, Finkelstein S, Kliger A, Finkelstein F. The BDI and the SF36: Which tool to use to screen for depression? Adv Perit Dial. 2003;19:159–162.
- Halen NV, Cukor D, Constantiner M, Kimmel PL. Depression and mortality in end-stage renal disease. Curr Psychiatry Rep. 2012;14:36–44.
- Newson MJ, Pope GR, Roberts EM, Lolait SJ, O'Carroll AM. Stress-dependent and gender-specific neuroregulatory roles of the apelin receptor in the hypothalamic-pituitary-adrenal axis response to acute stress. J Endocrinol. 2013;216:99–109.
- Lee DK, Cheng R, Nguyen T, et al. Characterization of apelin, the ligand for the APJ receptor. J Neurochem. 2000;74:34–41.
- Mesmin C, Fenaille F, Becher F, Tabet JC, Ezan E. Identification and characterization of apelin peptides in bovine colostrum and milk by liquid chromatography-mass spectrometry. J Proteome Res. 2011;10:5222–5231.
- O'Carroll AM, Lolait SJ, Harris LE, Pope GR. The apelin receptor APJ: Journey from an orphan to a multifaceted regulator of homeostasis. J Endocrinol. 2013;219:R13–R35.
- Fox SE. The functions of the limbic system. In: Greger R, Windhorst U, eds. Comprehensive Human Physiology: From Cellular Mechanisms to Integration. Germany: Springer; 1996:355–374.
- Reaux A, De Mota N, Skultetyova I, et al. Physiological role of a novel neuropeptide, apelin, and its receptor in the rat brain. J Neurochem. 2001;77:1085–1096.
- Lv SY, Qin YJ, Wang HT, Xu N, Yang YJ, Chen Q. Centrally administered apelin-13 induces depression-like behavior in mice. Brain Res Bull. 2012;88:574–580.
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571.
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. J Consult Clin Psychol. 1988;56:893–897.
- Sacks CR, Peterson RA, Kimmel PL. Perception of illness and depression in chronic renal disease. Am J Kidney Dis. 1990;15:31–39.
- Guney I, Solak Y, Atalay H, et al. Comparison of effects of automated peritoneal dialysis and continuous ambulatory peritoneal dialysis on health-related quality of life, sleep quality, and depression. Hemodial Int. 2010;14:515–522.
- Wuerth D, Finkelstein SH, Ciarcia J, Peterson R, Kliger AS, Finkelstein FO. Identification and treatment of depression in a cohort of patients maintained on chronic peritoneal dialysis. Am J Kidney Dis. 2001;37:1011–1017.
- Dong J, Pi HC, Xiong ZY, et al. Depression and Cognitive Impairment in Peritoneal Dialysis: A Multicenter Cross-sectional Study. Am J Kidney Dis. 2016; 67:111–118.
- Martínez-Sanchis S, Bernal MC, Montagud JV, Abad A, Crespo J, Pallardó LM. Quality of life and stressors in patients with chronic kidney disease depending on treatment. Span J Psychol. 2015;18:E25.
- Kutner NG, Fair PL, Kutner MH. Assessing depression and anxiety in chronic dialysis patients. J Psychosom Res. 1985;29:23–31.
- Pop-Jordanova ND, Polenakovic MH. Psychological characteristics of patients treated by chronic maintenance hemodialysis. Int J Artif Organs. 2013;36:77–86.
- Bossola M, Ciciarelli C, Di Stasio E, et al. Symptoms of depression and anxiety over time in chronic hemodialysis patients. J Nephrol. 2012;25:689–698.
- Cukor D, Coplan J, Brown C, et al. Depression and anxiety in urban hemodialysis patients. Clin J Am Soc Nephrol. 2007;2:484–490.
- Patel A. Review: The role of inflammation in depression. Psychiatr Danub. 2013;2:216–223.
- Zeugmann S, Quante A, Heuser I, Schwarzer R, Anghelescu I. Inflammatory biomarkers in 70 depressed inpatients with and without the metabolic syndrome. J Clin Psychiatry. 2010;71:1007–1016.
- Kim YK, Na KS, Shin KH, Jung HY, Choi SH, Kim JB. Cytokine imbalance in the pathophysiology of major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1044–1053.
- Kalender B, Ozdemir AC, Koroglu G. Association of depression with markers of nutrition and inflammation in chronic kidney disease and end-stage renal disease. Nephron Clin Pract. 2006;102:c115–c121.
- Ko GJ, Kim MG, Yu YM, Jo SK, Cho WY, Kim HK. Association between depression symptoms with inflammation and cardiovascular risk factors in patients undergoing peritoneal dialysis. Nephron Clin Pract. 2010;116:c29–c35.
- Li ZJ, An X, Mao HP, et al. Association between depression and malnutrition-inflammation complex syndrome in patients with continuous ambulatory peritoneal dialysis. Int Urol Nephrol. 2011;43:875–882.
- Boulware LE, Liu Y, Fink NE, et al. Temporal relation among depression symptoms, cardiovascular disease events, and mortality in end-stage renal disease: Contribution of reverse causality. Clin J Am Soc Nephrol. 2006;1:496–504.
- Kalender B, Dervisoglu E, Sengul E, et al. Depression, nutritional status, and serum cytokines in peritoneal dialysis patients: Is there a relationship? Perit Dial Int. 2007;27:593–595.
- Chilcot J, Davenport A, Wellsted D, Firth J, Farrington K. An association between depressive symptoms and survival in incident dialysis patients. Nephrol Dial Transplant. 2011;26:1628–1634.
- Preljevic VT, Østhus TB, Sandvik L, et al. Psychiatric disorders, body mass index and C-reactive protein in dialysis patients. Gen Hosp Psychiatry. 2011;33:454–461.
- Dogan E, Erkoc R, Eryonucu B, Sayarlioglu H, Agargun MY. Relation between depression, some laboratory parameters, and quality of life in hemodialysis patients. Ren Fail. 2005;27:695–699.
- Małyszko J, Małyszko JS, Koźminski P, Myśliwiec M. Apelin and cardiac function in hemodialyzed patients: Possible relations? Am J Nephrol. 2006;26:121–126.
- Kleinz MJ, Davenport AP. Emerging roles of apelin in biology and medicine. Pharmacol Ther. 2005;107:198–211.
- De Mota N, Reaux-Le Goazigo A, El Messari S, et al. Apelin, a potent diuretic neuropeptide counteracting vasopressin actions through inhibition of vasopressin neuron activity and vasopressin release. Proc Natl Acad Sci U S A. 2004;101:10464–10469.
- Szokodi I, Tavi P, Foldes G, et al. Apelin, the novel endogenous ligand of the orphan receptor APJ, regulates cardiac contractility. Circ Res. 2002;91:434–440.
- Weir RA, Chong KS, Dalzell JR, et al. Plasma apelin concentration is depressed following acute myocardial infarction in man. Eur J Heart Fail. 2009;11:551–558.
- Li Y, Chen J, Bai B, Du H, Liu Y, Liu H. Heterodimerization of human apelin and kappa opioid receptors: Roles in signal transduction. Cell Signal. 2012;24:991–1001.
- Carlezon WA, Jr, Béguin C, DiNieri JA, et al. Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J Pharmacol Exp Ther. 2006;316:440–447.
- Wadenberg ML. A review of the properties of spiradoline: A potent and selective kappa-opioid receptor agonist . CNS Drug Rev. 2003;9:187–198.
- Yu S, Zhang Y, Li MZ, et al. Chemerin and apelin are positively correlated with inflammation in obese type 2 diabetic patients. Chin Med J (Engl). 2012;125:3440–3444.
- García-Díaz D, Campión J, Milagro FI, Martínez JA. Adiposity dependent apelin gene expression: Relationships with oxidative and inflammation markers. Mol Cell Biochem. 2007;305:87–94.
- Fernandez M. Apelin signaling modulates splanchnic angiogenesis and portosystemic collateral vessel formation in rats with portal hypertension. J Hepatol. 2009;50:296–305.
