Abstract
Background: Cytokines are essential mediators of immune response. Chronic renal failure patients suffer from chronic inflammation that results from factors such as impaired renal function, accumulation of uremic toxins and bio incompatibility of dialyzer membranes. These patients are also at increased risk of cardiovascular diseases. We have evaluated cytokines, adipocytokines and inflammatory markers in patients with chronic renal failure undergoing hemodialysis (HD) and continuous ambulatory peritoneal dialysis (CAPD).
Material and methods: We have determined serum tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), high-sensitivity C-reactive protein (hsCRP), leptin and ghrelin levels of chronic renal failure patients treated with either HD (n = 20) or CAPD (n = 20). TNF-α, IL-6, ghrelin and leptin measurements were performed by commercially available kits based on enzyme-linked immunosorbent assay (ELISA) method. hsCRP levels were determined by turbidimetric methods.
Results: Serum TNF-α and IL-6 levels of patients on HD were significantly higher than those of the ones on CAPD (p < 0.05). Ghrelin, leptin and hsCRP concentrations were similar in both groups.
Conclusions: We can conclude that cytokine production is more obvious in HD process.
Introduction
Inflammation is common in chronic renal failure (CRF). There are reports demonstrating that chronic inflammation is one of the underlying mechanisms of morbidity and mortality in CRF. Uremia causes disturbances in immune system and increases the risk of infection. Repeated dialysis applications cause decrease in leukocyte activation and lead ultimately to an increase in cytokine production in patients with CRF.Citation1
In CRF, chronic inflammation is mediated by cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6). Both cytokines are related with the regulation of acute-phase reactants, carbohydrate and lipid metabolism and risk for the development of cardiovascular diseases (CVD) in individuals with renal dysfunction.Citation2 It has been shown that hsCRP was the determinant of CVD that is the major cause of mortality and morbidity in CRF.Citation3,Citation4 CRP has been used in the determination of acute infections, tissue damage and inflammation for a long time.Citation5 In recent years, a number of studies have been published indicating that CRP has proatherogenic features.Citation6–9
Inflammatory events in CRF also bring a series of complications. Cytokines, such as IL-6 and TNF-α, have significant impact on function and release of neurotransmitters by affecting central nervous system (CNS). As a result of the effects of cytokines on CNS, malnutrition is commonly observed in collaboration with inflammation. Increased release of proinflammatory cytokines in CRF cause loss of appetite in patients with CRF, by affecting the production of hormones related with central regulation of appetite. Ghrelin and leptin are hormones that have critical role in regulation of appetite and also related with inflammation.Citation10–12
Hemodialysis (HD) and continuous ambulatory peritoneal dialysis (CAPD) are techniques commonly used in maintenance of patients with CRF until renal transplantation. Although there are many reports about these two methods in patients with end-stage renal disease (ESRD), it is still uncertain which one contributes to prolonged survival. Some of the authors suggested that HD was superior to CAPD, while some others preferred CAPD. Reports indicating that they have similar effects on survival were also available.Citation13
In our study, We aimed to evaluate cytokines, adipocytokines and inflammatory markers in patients with CRF undergoing CAPD and HD.
Materials and methods
Subjects
A total of 40 patients with CRF were contributed in this study. Among these 40 participants, 20 were on chronic hemodialysis (HD) and 20 on continuous ambulatory peritoneal dialysis (CAPD). Age and sex distribution of these two groups were similar. Patients with diabetes, hypertension, malignant diseases, on immunosuppressive therapy were excluded from the study. Polysulfone membranes were used in HD process and HD was carried out for three sessions (4 h/sessions) per week. In CAPD process, the same conventional PD solutions (1.36%, 2.27% and 3.86% glucose; lactate buffer; 1.25% calcium) from Baxter Healthcare (Deerfield, IL) were used.
Sample collection and biochemical analysis
Fasting blood samples of the participants were obtained into vacuated blood collection tubes with no additives before dialysis. Serum portions were separated after centrifugation for 15 min at 2000×g. Serum levels of TNF-α (AssayMax human TNF-α, ET2010–1, AssayPro), IL-6 (AssayMax Human IL-6, EI1006–1, AssayPro), leptin (MyBioSource Leptin, human, MBS020274, MyBioSource) and ghrelin (SpiBio Acylated human ghrelin, A05106, Bertin Pharma, France) were determined with enzyme-linked immunosorbent assay (ELISA) technique. Intra- and interassay CV values for TNF-α assay were 3.0% and 8.0%. Minimum detectable concentration was 5 pg/mL. Intra- and interassay CV values for IL-6 assay were 5,1% and 7%. Minimum detectable concentration was <10 pg/mL. Intra- and inter-assay CV values for leptin assay were 3.6% v and 5.2%. Minimum detectable concentration was 0.1 ng/mL. Intra- and interassay CV values for acylated ghrelin assay were 6.2% v and 6.7%. Minimum detectable concentration was 0.8 pg/mL. hsCRP quantification was performed by using immunoturbimetric method (Dade Behring, Siemens) on Dimension Xpand (Siemens, Germany). Intra- and interassay CV values for hsCRP assay were 2.0% and 2.7%. Minimum detectable concentration was 0.175 mg/L.
Statistical analysis
Statistical analysis was performed by using SPSS for Windows (version 15.0), statistical software package (SPSS Inc., Chicago, IL). Kolmogorov–Smirnov test was used to analyze the distribution of numerical data. Chi-square test was used to assess the relationship between HD and CAPD groups for categorical variables. All numerical data are presented as mean ± SD. Mann–Whitney U-test was used for comparison between groups and p < 0.05 was assigned as statistically significant. Spearman's correlation analysis was also performed for the evaluation of the relations between the parameters.
Results
Demographic characteristics and biochemical parameters of patients undergoing hemodialysis and continuous ambulatory peritoneal dialysis were presented in . TNF-α and IL-6 levels were significantly higher in HD group compared to the CAPD group (p < 0.05) (). However, ghrelin, leptin and hsCRP concentrations were similar in both groups (p > 0.05). There was no significant correlation between the tested parameters.
Figure 1. IL-6 and TNF-α levels of patients undergoing hemodialysis and continuous ambulatory peritoneal dialysis.
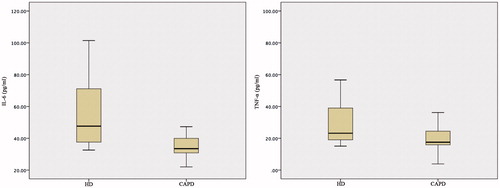
Table 1. Demographic characteristics and biochemical parameters of patients undergoing hemodialysis and continuous ambulatory peritoneal dialysis.
Discussion
In our study, cytokines, inflammatory markers and adipocytokines, such as IL-6, TNF-α, hsCRP, ghrelin and leptin, were evaluated in patients CRF on maintenance either with HD or CAPD. Among these parameters, IL-6 and TNF-α levels were significantly higher in HD group compared to the CAPD group, while ghrelin, leptin and hsCRP concentrations were similar in both groups.
In CRF, incidence of CVD is seen 10 to 30 times more than the normal population. In recent reports; inflammation, hyperuricemia and oxidative stress were suggested as the underlying causes of increased risk of CVD in CRF.Citation14 In the literature, there is limited number of studies that evaluate cytokine levels in patient with CRF undergoing CAPD and HD. Tbahriti et al. evaluated pro-inflammatory cytokines as TNF-α, IL-1β, IL-6 in HD and CAPD groups and reported that these cytokines were increased in HD group compared to CAPD groupCitation1 and these results are in agreement with our findings. While Borazan et al. observed that TNF-α, IL-10, IL-6 levels were similar in both HD and CAPD groups. Citation3
Immune dysfunction is common in CRF including immune cell reactivity, phenotypic changes in receptors and changes in expression of cell surface receptors. These changes cause an increase in uremic toxins and deterioration of renal excretory functions, besides altering the bioavailability of dialysis membranes. In relation with these changes in renal function, as a result of increased cytokine production and/or decreased renal clearance, cytokine levels increase in patients with CRF compared to healthy individuals.Citation1,Citation15 Increased cytokine production being more noticeable in HD compared to CAPD may be due to invasive arteriovenous fistula and synthetic membranes used in HD, which are more prone to inflammation whereas peritoneum used in CAPD is a natural membrane.
Previous reports demonstrated that hsCRP, which is an inflammatory marker and plays a role in cytokine release, was associated with increased rate of mortality in CRF related with CVD. hsCRP plays a critical role in endothelial injury, and pathogenesis of atherosclerosis, besides being an inflammation marker.Citation16–18 Borazan et al. evaluated CRP levels in HD and CAPD and stated that there was no significant difference between both groups.Citation3 We have also observed similar levels of hsCRP in both groups. In contrast to increased cytokines, such as TNF-α, and IL-6 in HD, no significant difference for hsCRP might be due to as a result of difference in the clearance of hsCRP and cytokines from dialysate membranes and peritoneum.
In CRF, inflammatory cytokines such as TNF-α and IL-6 cause changes in the release and function of the neurotransmitters by affecting the CNS. Due to these changes; anorexia, increased energy consumption, depletion of the protein stores, destruction in adipose and muscle tissues occur.Citation10 Leptin and ghrelin are neurohormones involved in appetite and energy metabolism and released from gastric mucosa. Ghrelin stimulates food intake, reduce energy consumption and lead to weight gain. Whereas leptin which is secreted by gastric epithelium and adipose tissue, reflects body fat stores, and stabilizes body weight by suppressing food intake and increasing energy consumption.Citation19 Besides the impact of these two peptide hormones on appetite, proinflammatory characteristics of these hormones were also reported in human and animal studies.Citation20–23 Both hormones are associated with the production of proinflammatory cytokines such as TNF-α and IL-1β. On the other hand, inflammation reduces serum ghrelin levels, while increasing leptin levels.Citation20 Depending on the relation of these hormones with inflammation, leptin and ghrelin were associated with CVD in patients with CRF. They are proatherogenic and related with vascular calcification and oxidative stress and these are the probable causes for their contribution in CVD.Citation24–26 When we searched medical databases, we could not find any study evaluating leptin and ghrelin levels in patients with CRF on maintenance with CAPD and HD. However, there are various reports about leptin or ghrelin levels in CRF.Citation10,Citation27–30 A large part of them covered the assessment of nutritional status and BMI with these peptide hormones. The study of Yildiz et al was the only one compatible with our study in terms of the parameters evaluated such as TNF-α, IL-6, hs-CRP and leptin in CAPD and HD groups.Citation31 However, the findings of this study were not compatible with our results. Yildiz et al. stated that there was no difference for serum TNF-α, IL-6, hs-CRP and leptin levels between HD and CAPD groups, but there was a strong correlation between these parameters and serum leptin levels. In our study, we evaluated serum leptin and ghrelin levels with TNF-α, IL-6 and hs-CRP in HD and CAPD groups. However, we did not observe any significant difference for leptin and ghrelin levels in both groups. Moreover, we did not find any significant correlation between these parameters and cytokine levels.
Our study has some limitations. Small sample size and unavailability of repeated measurements after dialysis process were the limitations. Insufficient budget for reagent costs were the main reason for these limitations.
We can conclude that inflammatory cytokines are significantly higher in HD compared to CAPD. HD process induces inflammation and this can be related with the increased risk of CVD in this population. Prospective studies with larger sample sizes and with repeated measurements demonstrating the effect of each sequence of dialysis process will clarify the exact relation between these markers and these dialysis modalities.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Tbahriti HF, Meknassi D, Moussaoui R, et al. Inflammatory status in chronic renal failure: The role of homocysteinemia and pro-inflammatory cytokines. World J Nephrol. 2013;2:31–37.
- Stompor T, Pasowicz M, Sullowicz W, et al. An association between coronary artery calcification score, lipid profile, and selected markers of chronic inflammation in ESRD patients treated with peritoneal dialysis. Am J Kidney Dis. 2003;41:203–211.
- Borazan A, Ustün H, Ustundag Y, et al. The effects of peritoneal dialysis and hemodialysis on serum tumor necrosis factor-alpha, interleukin-6, interleukin-10 and C-reactive-protein levels. Mediators Inflamm. 2004;13:201–204.
- Rifai N, Ridker PM. Proposed cardiovascular risk assessment algorithm using high-sensitivity C-reactive protein and lipid screening. Clin Chem. 2001;47:28–30.
- Kaylan N. Kardiyovasküler bir risk faktörü olarak CRP. AII Konseyi Bülten. 2005;9:1–4.
- Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102:2169–2172.
- Pasceri V, Chang J, Willerson JT, et al. Modulation of C-reactive protein-mediated monocyte chemoattractant protein-1 induction in human endothelial cells by antiatherosclerosis drugs. Circulation. 2000;103:2531–2534.
- Verma S, Li SH, Badiwala MV, et al. Endothelin antagonism and interleukin-6 inhibition attenuate the proatherogenic effects of C-reactive protein. Circulation. 2002;105:1890–1896.
- Verma S, Wang CH, Li SH, et al. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. 2002;106:913–919.
- Barros AF, Moraes C, Pinto MB, Lobo JC, Mafra D. Is there association between acyl-ghrelin and inflammation in hemodialysis patients? J Bras Nefrol. 2013;35:120–126.
- Striz I, Trebichavsky I. Calprotectin-a pleiotropic molecule in acute and chronic inflammation. Physiol Res. 2004;53:245–253.
- Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397.
- Yang F, Khin LW, Lau T, et al. Hemodialysis versus peritoneal dialysis: A comparison of survival outcomes in south-east asian patients with end-stage renal disease. PLoS One. 2015;10:1–10.
- Helal I, Smaoui W, Hamida FB, et al. Cardiovascular risk factors in hemodialysis and peritoneal dialysis patients. Saudi J Kidney Dis Transpl. 2010;21:59–62.
- Rysz J, Banach M, Cialkowska-Rysz A, et al. Blood serum levels of IL-2, IL-6, IL-8, TNF-alpha and IL-1beta in patients on maintenance hemodialysis. Cell Mol Immunol. 2006;3:151–154.
- Gad MZ, El-Mesallamy HO, Sanad EF. hsCRP, sICAM-1 and TAFI in hemodialysis patients: linking inflammation and hypofibrinolysis to cardiovascular events. Kidney Blood Press Res. 2008;31:391–397.
- Koenig W, Khuseyinova N. Biomarkers of atherosclerotic plaque instability and rupture. Arterioscler Thromb Vasc Biol. 2007;27:15–26.
- Urquhart BL, House AA. Assessing plasma total homocysteine in patients with end-stage renal disease. Perit Dial Int. 2007;27:476–488.
- Erdemir G, Ozkan TB, Ozgur T, et al. Helicobacter pylori infection in children: Nutritional status and associations with serum leptin, ghrelin, and IGF-1 levels. Helicobacter. 2015. [Epub ahead of print]. doi: 10.1111/hel.12288.
- Cobanoglu N, Galip N, Dalkan C, et al. Leptin, ghrelin and calprotectin: inflammatory markers in childhood asthma? Multidiscip Respir Med. 2013;8:6225.
- Shore SA, Schwartzman IN, Mellema MA, et al. Effect of leptin on allergic airway responses in mice. J Allergy Clin Immunol. 2005;115:103–109.
- Matsuda K, Nishi Y, Okamatsu Y, et al. Ghrelin and leptin: A link between obesity and allergy? J Allergy Clin Immunol. 2006;117:705–706.
- Guler N, Kirerleri E, Ones U, et al. Leptin: Does it have any role in childhood asthma? J Allergy Clin Immunol. 2004;114:254–259.
- Bouloumie A, Drexler HC, Lafontan M, et al. Leptin, the product of ob gene, promotes angiogenesis. Circ Res. 1998;83:1059–1066.
- Parhami F, Tintut Y, Ballard A, et al. Leptin enhances the calcification of vascular cells: artery wall as a target of leptin. Circ Res. 2001;88:954–960.
- Bouloumie A, Marumo T, Lafontan M, et al. Leptin induces oxidative stress in human endothelial cells. FASEB J. 1999;13:1231–1238.
- Dervisevic A, Subo A, Avdagic N, et al. Elevated serum leptin level is associated with body mass index but not with serum C-reactive protein and erythrocyte sedimentation rate values in hemodialysis patients. Mater Sociomed. 2015;27:99–103.
- Montazerifar F, Karajibani M, Hassanpour Z, et al. Study of serum levels of leptin, C-reactive protein and nutritional status in hemodialysis patients. Iran Red Crescent Med J. 2015;17:1–5.
- Szczepańska M, Szprynger K, Mazur B, et al. Plasma ghrelin levels in children with chronic renal failure on peritoneal dialysis. Perit Dial Int. 2007;27:61–66.
- Bilen Y, Cankaya E, Bilen N, et al. Peritonitis incidence was correlated with duration of peritoneal dialysis rather than leptin or neutrophil to lymphocyte (n/L) ratio in peritoneal dialysis patients. Eurasian J Med. 2014;46:145–150.
- Yildiz G, Yilmaz A, Nur N, et al. Leptin as an inflammatory marker in dialysis patients. Turkiye Klinikleri J Med Sci. 2010;30:1482–1486.
