Abstract
Background: Although vascular calcification in end-stage renal disease (ESRD) represents a ubiquitous human health problem, effective therapies with limited side effects are still lacking, and the precise mechanisms are not fully understood. The Nrf-2/ARE pathway is a pivotal to regulate anti-oxidative responses in vascular calcification upon ESRD. Although Nrf-2 plays a crucial role in atherosclerosis, pulmonary fibrosis, and brain ischemia, the effect of Nrf-2 and oxidative stress on vascular calcification in ESRD patients is still unclear. The aim of this research was to study the protective role of hydrogen peroxide in vascular calcification and the mechanism of Nrf-2 and oxidative stress on vascular calcification.
Materials and methods: Here we used the rat vascular smooth muscle cell model of β-glycerophosphate-induced calcification resembling vascular calcification in ESRD to investigate the therapeutic effect of 0.01 mM hydrogen peroxide on vascular calcification and further explores the possible underlying mechanisms.
Results: Our current report shows the in vitro role of 0.01 mM hydrogen peroxide in protecting against intracellular ROS accumulation upon vascular calcification. Both hydrogen peroxide and sulforaphane pretreatment reduced ROS production, increased the expression of Nrf-2, and decreased the expression of Runx2 following calcification.
Conclusion: Our study demonstrates that 0.01 mM hydrogen peroxide can effectively protect rat aortic vascular smooth muscle cells against oxidative stress by preventing vascular calcification induced ROS production through Nrf-2 pathway. These data might define an antioxidant role of hydrogen peroxide in vascular calcification upon ESRD.
Introduction
End-stage renal disease (ESRD) is a ubiquitously health problem all over the world but effective therapies with limited side effects are still lacking.Citation1–3 Vascular calcification is one of the leading causes for ESRD patients.Citation2 As characterized by no calcification, lipid, cholesterol deposits and luminal stenosis in arterial smooth muscle layer, vascular calcification is involved in the complex process that vascular smooth muscle cells could transform to osteogenesis cells.Citation4–6 The uremic toxins, calcium phosphate metabolic disorders, inflammatory factors, endoplasmic reticulum stress, activated vitamin D in serum, and blood dialysis could induce vascular calcification in ESRD patients.Citation7–11 Therefore, to explore novel mechanism of vascular calcification in ESRD patients is of great significance.
Recently, people have gradually recognized the vital role of oxidative stress in vascular calcification. Oxidative stress is due to the imbalance between the generation and clearance of free radicals by enzymes.Citation12 Nuclear transcription factor NF-E2 related factor 2 (nuclear factor-erythroid2-related factor-2, Nrf-2) is one of the most important intracellular antioxidant factor to regulate oxidative stress.Citation13 Activation of Nrf-2 could protect cells from apoptosis, inflammation through regulating oxidative stress.Citation14 Although Nrf-2 plays a crucial role in atherosclerosis, pulmonary fibrosis, and brain ischemia, the effect of Nrf-2 and oxidative stress on vascular calcification in ESRD patients is still unclear.
To delve into the effect and mechanism of Nrf-2 and oxidative stress on vascular calcification, the aim of this research was to study the protective role of hydrogen peroxide in vascular calcification. Our results showed that hydrogen peroxide might prevent vascular calcification induced ROS production by regulating Nrf-2 pathway. These data might define an antioxidant role of hydrogen peroxide in vascular calcification and set the new stage for therapy of vascular calcification in ESRD.
Materials and methods
Reagents
Sulforaphane (SFN) (#S6317, Sigma-Aldrich, St. Louis, MO), hydrogen peroxide (#H1009), β-glycerophosphate (#G6376), L-ascorbic acid (#A5960), and dexamethasone (#D4902) were purchased from Sigma-Aldrich (St. Louis, MO).
Cell culture
Rat aortic vascular smooth muscle cells (RASMCs) was obtained from ScienCell Research Laboratories (Carlsbad, CA) and maintained following the supplier’s instructions. These cells were grown in DMEM containing 10% FBS, 100 units/mL penicillin, and 100 μg/mL streptomycin in a 95% air, 5% CO2 humidified atmosphere at 37 °C. Following to the study of Mody et al., the calcification medium was made from 250 mL DMEM (containing 10% FBS, 100 units/mL penicillin, and 100 μg/mL streptomycin), 11 mg L-ascorbic acid, 770 mg β-glycerophosphate, and 10−8 M dexamethasone.Citation15
MTT assay
To find the effective dose of hydrogen peroxide, RASMCs were pretreated with hydrogen peroxide of various concentrations (0.01 mM, 0.02 mM, 0.05 mM, and 0.08 mM) for 2 h. Then the cells were treated with calcification medium for 48 h and normal saline was used as a control, 5 wells were included in each group. The cell survival was assessed by MTT assay kit (#C0009, Beyotime, Shanghai, China) after 48-h calcification. Then the absorbance at 570 nm was measured by SpectraMax M5 (Molecular Devices), using wells without cells as blanks. All experiments were performed in triplicate. The survival rate of the hydrogen peroxide-treated RASMCs was generated as the % cell growth rate, using the following formula: % survival rate = A570 of treated cells/A570 of control cells ×100%.
Cell morphology
To observe the cell morphology, RASMCs were pretreated with hydrogen peroxide of various concentrations (0.01 mM, 0.02 mM, 0.05 mM, and 0.08 mM) for 2 h. And the cells were treated with calcification medium for 48 h and normal saline was used as a control.
Reactive oxygen species assay
DCFH-DA (#S0033, Beyotime) is a probe can easily load into the cell. When DCFH-DA loaded into the cell, intracellular reactive oxygen species could oxidize the non-fluorescent DCFH to fluorescent DCF. Then the green fluorescence of DCF can indicate the level of intracellular reactive oxygen species. RASMCs were seeded at a density of 5 × 103 per well in 96-well plates (Costar Corning, Rochester, NY). After incubation overnight, cells were pretreated with 0.01 mM hydrogen peroxide for 2 h or 5 μM SFN for 6 h upon 12-, 24-, and 48-h calcification. Five wells were included in each group. After a 15-min incubation, fluorescence was measured by a fluorometer (BioTek, Winooski, VT).
Isolation of nuclear extracts
Nuclear extracts were isolated following the instruction of NE-PER Nuclear and Cytoplasmic Extraction Reagents Kit (#78833, Pierce/Thermofisher Scientific, USA). Cells were washed by suspending the cell pellet with phosphate-buffered saline. We added the CER I buffer and CER II buffer to swell the cells on ice for 10 min and then vortexes for 10 s. Subsequently, samples were centrifuged for 10 s and the supernatant fraction was discarded. Pellets were suspended in NER buffer and incubated on ice for 40 min for high-salt extraction. Cellular debris was removed by centrifugation for 10 min at 16,000 g and the supernatant fraction (containing DNA-binding proteins) was stored at −80 °C until use. Protein concentrations were determined by the BCA Protein Assay Kit (#P0010, Beyotime).Citation16
Western blotting analysis
The samples from cells were lysed and quantified. About 20 μg of protein from RASMCs were mixed with an equal volume of 2× SDS sample buffer, boiled for 5 min, and then separated by 10% SDS-polyacrylamide gel electrophoresis (PAGE). After electrophoresis, proteins were transferred to nitrocellulose membranes (Thermofisher Scientific). Membranes were incubated with primary antibodies against β-actin (#60008, Proteintech, China), PCNA (#10205, Proteintech), Nrf-2 (#16396, Proteintech), HO-1(#10701, Proteintech), and Runx2 (Runt-related transcription factor-2) (#20700, Proteintech). Western blotting rabbit polyclonal antibodies, mouse polyclonal antibodies, and goat polyclonal antibodies were obtained from Proteintech. Signals were visualized using an enhanced chemiluminescence detection kit (Millpore, USA). The protein signal was quantified by scanning densitometry using Quantity One imaging analysis (GE, USA).
Statistical analysis
Statistical analysis was performed with the SPSS software system (SPSS for Windows, version 13.0; SPSS Inc., Chicago, IL). Parametric data were statistically analyzed by one-way ANOVA followed by post hoc tests. Differences in non-parametric data were evaluated by the Mann–Whitney U test. Data were expressed as means ± SD. A significant difference was defined as p < 0.05.
Results
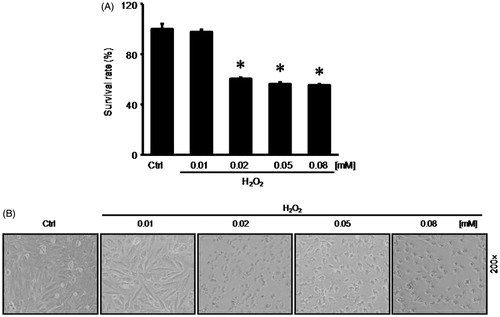
0.01 mM hydrogen peroxide had no effect on RASMC survival
To elucidate the effect of hydrogen peroxide on RASMCs, we pretreated RASMCs with hydrogen peroxide at different concentrations (0.01 mM, 0.02 mM, 0.05 mM, and 0.08 mM) for 2 h. During the 48-h incubation with calcification medium, the 0.02 mM hydrogen peroxide reduced the survival rate to 60.41 + 1.14%; the 0.05 mM hydrogen peroxide reduced the survival rate to 56.37 + 1.40%; the 0.08 mM hydrogen peroxide reduced the survival rate to 55.18 + 1.07%. However, 0.01 mM hydrogen peroxide had no significant effect on RASMCs survival rate (). Then we further observed the cellular morphology of RASMCs following hydrogen peroxide treatment. We found that RASMCs kept normal cell shape upon 0.01 mM hydrogen peroxide treatment ().
Figure 1. 0.01 nM hydrogen peroxide had no effect on RASMCs survival. To elucidate the effect of hydrogen peroxide on RASMCs, before the 48-h incubation with calcification medium, we pretreated RASMCs with hydrogen peroxide at different concentrations (0.01 mM, 0.02 mM, 0.05 mM, and 0.08 mM) for 2 h. (A) MTT assay to detect cell survival. (B) Cellular morphology of RASMCs following hydrogen peroxide treatment. Data are means ± SD; *p < 0.05 versus normal control.
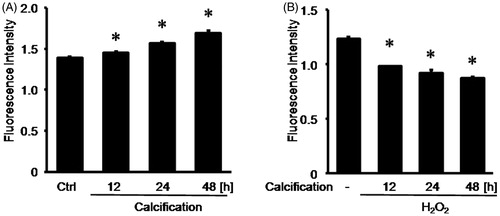
Hydrogen peroxide reduced ROS production in RASMCs upon calcification
We respectively treated RASMCs with calcification medium for 12 h, 24 h, and 48 h. Then we observed that the fluorescence intensity indicating ROS production was respectively increased to 1.45 ± 0.02 by 12-h calcification, 1.57 ± 0.02 by 24-h calcification, and 1.69 ± 0.03 by 48-h calcification, compared with 1.39 ± 0.01 in normal control group (). However, 0.01 mM hydrogen peroxide pretreatment respectively decreased the fluorescence intensity indicating ROS production to 0.98 ± 0.01 by 12-h calcification, 0.92 ± 0.03 by 24-h calcification, and 0.87 ± 0.02 by 48-h calcification, compared with 1.23 ± 0.02 in normal control group ().
Figure 2. Hydrogen peroxide reduced ROS production in RASMCs upon calcification. (A) The fluorescence intensity indicating ROS production was detected by reactive oxygen species assay following calcification. (B) The fluorescence intensity indicating ROS production was detected by reactive oxygen species assay following hydrogen peroxide pretreatment and calcification. To make sure if calcification could increase ROS production in a time-dependent manner, one-way ANOVA was performed to compare these groups. Data are means ± SD; *p < 0.05 versus normal control.
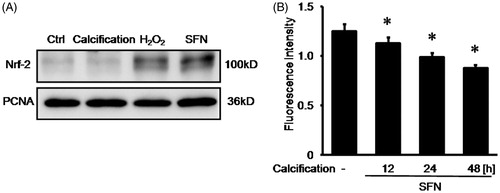
SFN up-regulated Nrf-2 expression in nuclear and reduced ROS production upon calcification
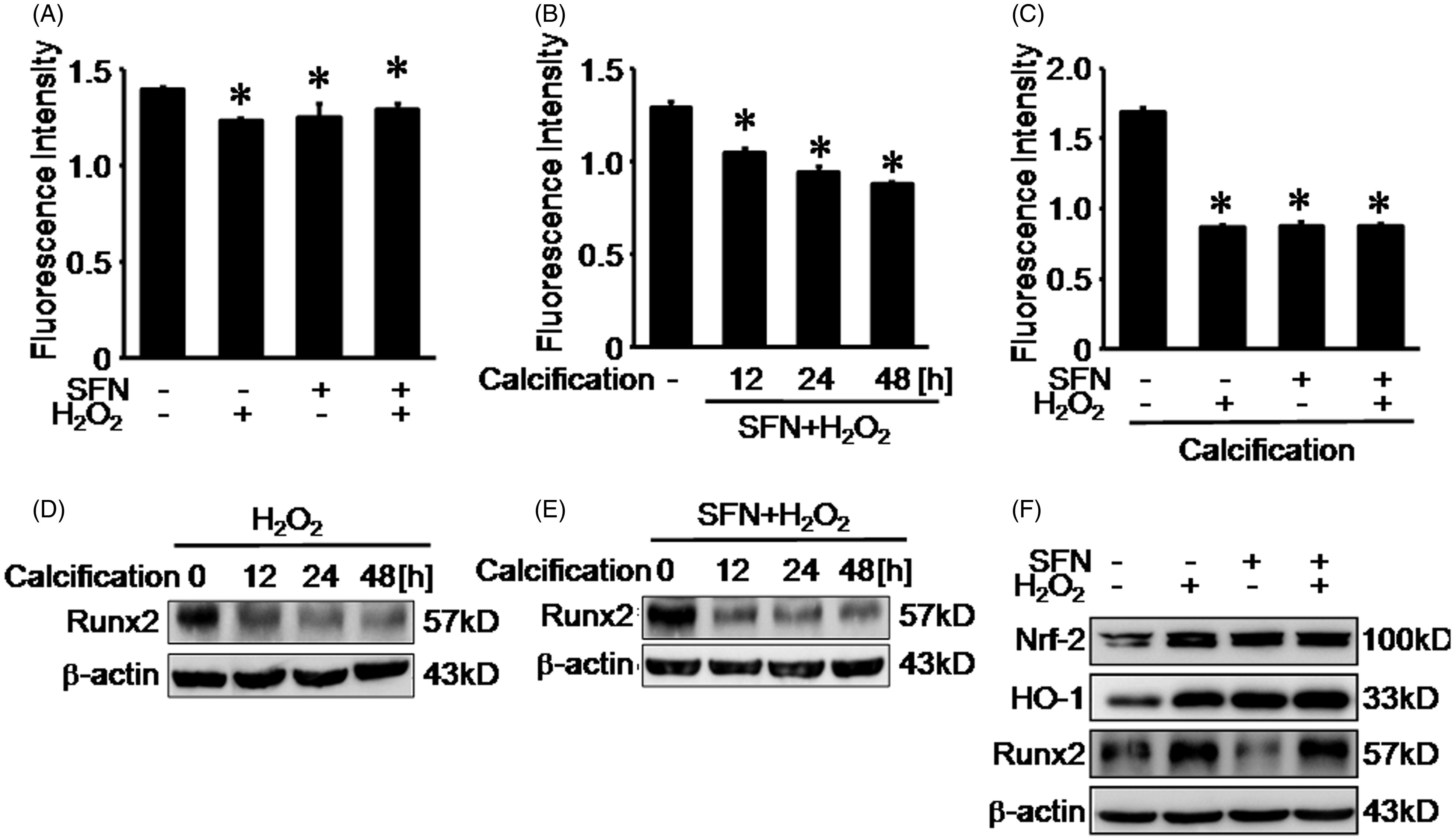
We detected the Nrf-2 expression in cell nuclear extracts. Both 0.01 mM hydrogen peroxide pretreatment and 5 μM SFN pretreatment could increase Nrf-2 expression in cell nuclear extracts. However, there was no significant change of Nrf-2 expression in cell nuclear extracts upon calcification, compared with normal control group (). Then we observed that the fluorescence intensity indicating ROS production after SFN pretreatment was respectively decreased to 1.13 ± 0.06 by 12-h calcification, 0.99 ± 0.04 by 24-h calcification, and 0.88 ± 0.03 by 48-h calcification, compared with 1.25 ± 0.07 in normal control group ().
Figure 3. SFN up-regulated the Nrf-2 expression in nuclear and reduced ROS production upon calcification. (A) Nrf-2 expression was detected by Western blotting. (B) ROS production was measured by reactive oxygen species assay following 5 μM SFN pretreatment and calcification. Data are means ± SD; *p < 0.05 versus normal control.
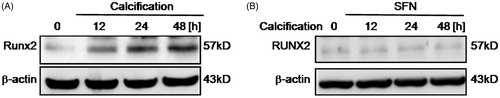
Calcification induced Runx2 expression then SFN decreased Runx2 expression following calcification
To further elucidate the effects of calcification on RASMCs, we detected expression of Runx-2 in RASMCs following calcification at 12 h, 24 h, and 48 h. We found that calcification for 24 h or 48 h could increase Runx2 expression in RASMCs (). However, after 5 μM SFN pretreatment, there was no significant change of Runx2 expression upon calcification, compared with normal control group ().
Hydrogen peroxide and SFN reduced ROS production upon calcification
Compared with normal control (1.39 ± 0.01), SFN decreased ROS fluorescence intensity to 1.23 ± 0.01, hydrogen peroxide reduced ROS fluorescence intensity to 1.25 ± 0.07 then both SFN and hydrogen peroxide pretreatment reduced ROS fluorescence intensity to 1.29 ± 0.03 (). After pretreated RASMCs with both hydrogen peroxide and SFN, we incubated the RASMCs with calcification medium for 12 h, 24 h, and 48 h. Compared with normal control (1.29 ± 0.03), hydrogen peroxide and SFN pretreatment decreased ROS fluorescence intensity to 1.04 ± 0.02 at 12-h calcification, 0.94 ± 0.03 at 24-h calcification, and 0.88 ± 0.01 at 48-h calcification (). Then we pretreated RASMCs with SFN and hydrogen following 48-h calcification. We observed that, compared with normal control (1.69 ± 0.03), SFN decreased ROS fluorescence intensity to 0.88 ± 0.03, hydrogen peroxide reduced ROS fluorescence intensity to 0.87 ± 0.02, and both SFN and hydrogen peroxide pretreatment reduced ROS fluorescence intensity to 0.88 ± 0.01 (). To further elucidate the effects of hydrogen peroxide on RASMCs upon calcification, we detected the expression of Runx2 in RASMCs following hydrogen peroxide pretreatment upon calcification at 12 h, 24 h, and 48 h. We found that hydrogen peroxide pretreatment significantly reduced Runx2 expression in RASMCs following calcification from 12 h to 48 h (). Then we pretreated RASMCs with both SFN and hydrogen following 12-, 24-, and 48-h calcification. We observed that, compared with normal control, both hydrogen peroxide and SFN treatment pretreatment significantly reduced Runx-2 expression in RASMCs following calcification from 12 h to 48 h (). Further we found that, compared with normal control, SFN treatment or hydrogen peroxide treatment and both SFN and hydrogen peroxide treatment up-regulated the expression of Nrf-2 and HO-1 expression in RASMCs. Compared with normal control, hydrogen peroxide treatment and both SFN and hydrogen peroxide treatment up-regulated the expression of Runx2 in RASMCs, however, SFN treatment down-regulated the expression of Runx2 in RASMCs ().
Figure 5. Hydrogen peroxide and SFN reduced ROS production upon calcification. (A) ROS production was measured by reactive oxygen species assay following 5 μM SFN treatment and 0.01 mM hydrogen peroxide treatment. (B) ROS production was measured by reactive oxygen species assay following both 5 μM SFN and 0.01 mM hydrogen peroxide treatment upon calcification from 12 h to 48 h. (C) ROS production was measured by reactive oxygen species assay following 5 μM SFN treatment and 0.01 mM hydrogen peroxide treatment upon calcification from 48 h. (D) Runx2 expression was detected by Western blotting following 0.01 mM hydrogen peroxide pretreatment and calcification. (E) Runx2 expression was detected by Western blotting following both 5 μM SFN and 0.01 mM hydrogen peroxide treatment upon calcification from 12 h to 48 h. (F) Runx2 expression was detected by Western blotting following 5 μM SFN treatment and 0.01 mM hydrogen peroxide treatment. Data are means ± SD; *p < 0.05 versus normal control.
Discussion
The damage, caused by hydrogen peroxide, to the balance between generation and clearance of free radicals in cells could induce oxidative stress.Citation17 As reported by Byon et al., hydrogen peroxide at the concentrations from 0.1 mM to 0.4 mM were innoxious to smooth muscle cells and could induce vascular calcification at a concentration dependence by up-regulating the expression of Runx2 through PI3K/AKT pathway.Citation15 However, Zucker et al. found that hydrogen peroxide on dosage more than 0.05 mM could increase the expression of Kruppel-like factor 9 (Klf9) and induce cell death by ROS accumulation, whereas hydrogen peroxide on dosage less than 0.05 mM had no effect on cell survival and activated Nrf-2-ARE pathway to clear intracellular ROS to keep the redox equilibrium in cells.Citation18 To elucidate the effect of hydrogen peroxide on RASMCs, we pretreated RASMCs with hydrogen peroxide at different concentrations (0.01 mM, 0.02 mM, 0.05 mM, and 0.08 mM) for 2 h. During the 48-h incubation with calcification medium, we observed that hydrogen peroxide on dosage more than 0.01 mM could inhibit RASMCs survival, whereas hydrogen peroxide on dosage of 0.01 mM had no effect on cell survival. Then our current study focused on the effect of 0.01 mM hydrogen peroxide on RASMC calcification.
Increased ROS production is one of the main reasons to break endogenous protective rule in vascular smooth muscle cells and redundant intracellular ROS can induce vascular complications.Citation19–21 We respectively treated RASMCs with calcification medium for 12 h, 24 h, and 48 h. And we found that the fluorescence intensity indicating ROS production was respectively increased to calcification, compared with normal control group, in a time-dependent manner. However, 0.01 mM hydrogen peroxide pretreatment respectively decreased the fluorescence intensity indicating ROS production. It indicated that hydrogen peroxide at the concentration of 0.01 mM could inhibit intracellular ROS production in RASMCs upon calcification.
Nrf-2/ARE pathway is an important antioxidant pathway against oxidative stress by removal from oxygen-free radicals.Citation22,Citation23 Nrf-2 are mainly binding to Kelch-like ECH-associated protein 1 (Keap1) and distributed in the cytoplasm.Citation24 Once activated by redundant ROS accumulation under oxidative stress, Nrf-2 could dissociate with Keap1 and translocate from cytoplasm to nuclear.Citation25 As a Nrf-2 agonist, SFN could induce Nrf-2 dissociation with Keap1 and translocation into nuclear to increase the expressions of antioxidants such as HO-1, NQO-1, and so on.Citation26,Citation27 In our study, we detected the Nrf-2 expression in cell nuclear extracts. Both 0.01 mM hydrogen peroxide pretreatment and 5 μM SFN pretreatment could increase Nrf-2 expression in cell nuclear extracts. Then we observed that the fluorescence intensity indicating ROS production after SFN pretreatment was respectively decreased following by calcification. Nrf-2 could interact with Runx-2 to inhibit association with OSE2 on osteocalcin to reduce the osteocalcin transcriptional activity. Runx-2, as one of osteoblastic differentiation marker, plays a critical role in cellular differentiation processes in osteoblasts.Citation28 Liu et al. have observed that hydrogen peroxide increased the expression of Runx2 and led vascular smooth muscle cells to transform to be osteogenesis cells upon oxidative stress.Citation29 To further elucidate the effects of calcification on RASMCs, we detected the expression of Runx-2 in RASMCs following calcification. We found that 0.01 mM hydrogen peroxide pretreatment significantly reduced Runx-2 expression in RASMCs following calcification. Then we pretreated RASMCs with both SFN and hydrogen following calcification. We observed that, compared with normal control, both 0.01 mM hydrogen peroxide and SFN treatment pretreatment significantly reduced Runx-2 expression in RASMCs following calcification. Further, we found that SFN treatment or hydrogen peroxide treatment and both SFN and hydrogen peroxide treatment up-regulated the expression of Nrf-2 and HO-1 expression in RASMCs. Compared with normal control, hydrogen peroxide treatment and both SFN and hydrogen peroxide treatment up-regulated the expression of Runx-2 in RASMCs, however, SFN treatment down-regulated the expression of Runx-2 in RASMCs. These results indicated that hydrogen peroxide might prevent vascular calcification induced ROS production by regulating Nrf-2 pathway. However, in current study, we just study the effects of 0.01 mM hydrogen peroxide on vascular calcification induced ROS production by regulating Nrf-2 pathway. It is interesting to further study the effects and mechanism of hydrogen peroxide at other dosages on vascular calcification induced ROS production by regulating Nrf-2 pathway.
In summary, we have observed an antioxidant protective activity of 0.01 mM hydrogen peroxide in a rat vascular smooth muscle cell model of β-glycerophosphate induced calcification resembling vascular calcification in ESRD. Our study demonstrates that 0.01 mM hydrogen peroxide can effectively protect RASMCs against oxidative stress by preventing vascular calcification induced ROS production through Nrf-2 pathway. Further preclinical and clinical studies using 0.01 mM hydrogen peroxide against oxidative stress may prove to be of great value in this field.
Funding information
This work was supported by National Natural Science Foundation of China (81270828, 81300618) and PhD Foundation of Sichuan Academy of Sciences & Sichuan Provincial People’s Hospital (2015BS05).
Disclosure statement
W.Z. and Y.L. carried out the experimental work and the data collection and interpretation. H.D. and Y.D. participated in the design and coordination of experimental work and acquisition of data. L.W. carried out the study design, the analysis and drafted the manuscript. The authors declare no financial or commercial conflict of interest.
References
- Disthabanchong S. Vascular calcification in chronic kidney disease: Pathogenesis and clinical implication. World J Nephrol. 2012;1:43–53.
- Sigrist MK, Taal MW, Bungay P, McIntyre CW. Progressive vascular calcification over 2 years is associated with arterial stiffening and increased mortality in patients with stages 4 and 5 chronic kidney disease. Clin J Am Soc Nephrol. 2007;2:1241–1248.
- Schurgers LJ. Vitamin K: Key vitamin in controlling vascular calcification in chronic kidney disease. Kidney Int. 2013;83:782–784.
- Tbahriti HF, Kaddous A, Bouchenak M, Mekki K. Effect of different stages of chronic kidney disease and renal replacement therapies on oxidant-antioxidant balance in uremic patients. Biochem Res Int. 2013;2013:358985.
- Gutteridge JM, Halliwell B. Antioxidants: Molecules, medicines, and myths. Biochem Biophys Res Commun. 2010;393:561–564.
- Ruiz S, Pergola PE, Zager RA, Vaziri ND. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 2013;83:1029–1041.
- Disthabanchong S. Lowering vascular calcification burden in chronic kidney disease: Is it possible? World J Nephrol. 2013;2:49–55.
- Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325.
- Ballanti P, Silvestrini G, Pisano S, et al. Medial artery calcification of uremic patients: A histological, histochemical and ultrastructural study. Histol Histopathol. 2011;26:191–200.
- Fang Y, Ginsberg C, Sugatani T, Monier-Faugere MC, Malluche H, Hruska KA. Early chronic kidney disease-mineral bone disorder stimulates vascular calcification. Kidney Int. 2014;85:142–150.
- New SE, Goettsch C, Aikawa M, et al. Macrophage-derived matrix vesicles: An alternative novel mechanism for microcalcification in atherosclerotic plaques. Circulation Res. 2013;113:72–77.
- Bartnicki P, Fijalkowski P, Majczyk M, Blaszczyk J, Banach M, Rysz J. Effect of methoxy polyethylene glycol-epoetin beta on oxidative stress in predialysis patients with chronic kidney disease. Med Sci Monitor. 2013;19:954–959.
- Xing HY, Cai YQ, Wang XF, et al. The cytoprotective effect of hyperoside against oxidative stress is mediated by the Nrf2-ARE signaling pathway through GSK-3β inactivation. PLoS One. 2015;10:e0145183
- Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116.
- Byon CH, Javed A, Dai Q, et al. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem. 2008;283:15319–15327.
- Kannan S, Pang H, Foster DC, Rao Z, Wu M. Human 8-oxoguanine DNA glycosylase increases resistance to hyperoxic cytotoxicity in lung epithelial cells and involvement with altered MAPK activity. Cell Death Different. 2006;13:311–323.
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247.
- Zucker SN, Fink EE, Bagati A, et al. Nrf2 amplifies oxidative stress via induction of Klf9. Mol Cell. 2014;53:916–928.
- King GL, Loeken MR. Hyperglycemia-induced oxidative stress in diabetic complications. Histochem Cell Biol. 2004;122:333–338.
- Jeong IK, King GL. New perspectives on diabetic vascular complications: the loss of endogenous protective factors induced by hyperglycemia. Diabetes Metab J. 2011;35:8–11.
- Matough FA, Budin SB, Hamid ZA, Alwahaibi N, Mohamed J. The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos Univ Med J. 2012;12:5–18.
- Jeong WS, Jun M, Kong AN. Nrf2: A potential molecular target for cancer chemoprevention by natural compounds. Antioxid Redox Signal. 2006;8:99–106.
- Chapple SJ, Siow RC, Mann GE. Crosstalk between Nrf2 and the proteasome: Therapeutic potential of Nrf2 inducers in vascular disease and aging. Int J Biochem Cell Biol. 2012;44:1315–1320.
- Giudice A, Arra C, Turco MC. Review of molecular mechanisms involved in the activation of the Nrf2-ARE signaling pathway by chemopreventive agents. Methods Mol Biol. 2010;647:37–74.
- Itoh K, Wakabayashi N, Katoh Y, et al. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86.
- Myzak MC, Dashwood RH. Chemoprotection by sulforaphane: Keep one eye beyond Keap1. Cancer Lett. 2006;233:208–218.
- Pan H, He M, Liu R, Brecha NC, Yu AC, Pu M. Sulforaphane protects rodent retinas against ischemia-reperfusion injury through the activation of the Nrf2/HO-1 antioxidant pathway. PLoS One. 2014;9:e114186.
- Hinoi E, Fujimori S, Wang L, Hojo H, Uno K, Yoneda Y. Nrf2 negatively regulates osteoblast differentiation via interfering with Runx2-dependent transcriptional activation. J Biol Chem. 2006;281:18015–18024.
- Liu H, Lu Q, Huang K. Selenium suppressed hydrogen peroxide-induced vascular smooth muscle cells calcification through inhibiting oxidative stress and ERK activation. J Cell Biochem. 2010;111:1556–1564.