Abstract
Long-term effects of high protein diets (HPDs) on kidneys are still not sufficiently studied. Irisin which increases oxygen consumption and thermogenesis in white fat cells was shown in skeletal muscles and many tissues. Nitric oxide synthases (NOS) are a family of enzymes catalyzing the production of nitric oxide (NO) from L-arginine. We aimed to investigate the effects of HPD, irisin and NO expression in kidney and relation of them with exercise and among themselves. Animals were grouped as control, exercise, HPD and exercise combined with HPD (exercise-HPD). Rats were kept on a HPD for 5 weeks and an exercise program was given them as 5 exercise and 2 rest days per week exercising on a treadmill with increasing speed and angle. In our study, while HPD group had similar total antioxidant capacity (TAC) levels with control group, exercise and exercise-HPD groups had lower levels (p < 0.05). Kidneys of exercising rats had no change in irisin or eNOS expression but their iNOS expression had increased (p < 0.001). HPD–E group has not been observed to cause kidney damage and not have a significant effect on rat kidney irisin, eNOS, or iNOS expression. Localization of irisin, eNOS, and iNOS staining in kidney is highly selective and quite clear in this study. Effects of exercise and HPD on kidney should be evaluated with different exercise protocols and contents of the diet. İrisin, eNOS, and iNOS staining localizations should be supported with various research studies.
Introduction
High protein diets (HPDs) are being proposed to people who exercise regularly for boost body performance. Short-term use of HPD along with exercise has been reported to have an effect on body performance. Yet, long-term adherence of such a diet is reported to cause dehydration, harm bone structure, hearth muscles, and kidney tissue.Citation1,Citation2 Long-term HPD consumption was observed to increase oxidative stress and therefore causing adverse effects on many organs including kidneys on animals during experiments.Citation3,Citation4 HPD led to metabolic acidosis on rats, causing negative effect on their kidneys.Citation1 But HPD long-term effects on kidneys are still not sufficiently studied.
Irisin is a protein that is cleaved from fibronectin type III domain 5 (FNDC5) mRNA via peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha), which is induced in bone, muscle, and fat tissues during exercise and heat changes. Irisin is a thermogenic glycoprotein consisting of 112 amino acids and converts white fat tissue to brown fat tissue.Citation5–7 Irisin which increases oxygen consumption and thermogenesis in white fat cells, led to decreased obesity, and increased total body energy consumption on rats fed with a high fat diet.Citation8,Citation9 Apart from skeletal muscles, irisin is reported to be also shown with immunoperoxidase method in nerve sheath, liver, pancreas, sweat glands, heart muscle, salivary glands, ligaments, leydig cells, fat tissues, and fetal tissues. FNDC5 mRNA is present primarily in muscle tissue but also in ovarian, testicular, bladder, kidney, hypophysis, and retina.Citation10–15
Nitric oxide (NO) is a signaling molecule synthesized from L-arginine by nitric oxide synthase (NOS) identified in skeletal muscles. It has three isoforms as endothelial NOS (eNOS), neuronal NOS (nNOS), and inducible NOS (iNOS).Citation16–18 NO which is synthesized in endothelial layer of blood vessels by eNOS and nNOS is necessary for normal physiological functions. During cases of tissue damage or excessive exercise, extra NO is produced via iNOS.Citation17,Citation18 The increased NO production causes to increase in other free radicals also produced from organic nitrogen, like peroxynitrite anion and hydroxyl. Such free radicals are quite active and can damage tissues and disable ion pumps, causing transport defects.Citation19 Increased NO can systematically damage to the other organs.Citation18 NO can also consume antioxidants and hamper their protective properties on organs against oxidative stress.Citation16–19 Mounting muscular activity by exercise leads to increased blood flow in muscles while decreasing it in some organs like kidneys and intestines.Citation18 While current literature focuses mainly on relation of NO with exercise and effects of them on muscle, their additive effects on kidney tissue has not been studied sufficiently.
Studies about relation of exercise and irisin have contradictory findings. Some studies report that irisin levels in blood increase in after exercise.Citation6,Citation20–23 Yet there are studies also reporting that exercise have no effect on irisin levels.Citation24,Citation25 Studies regarding irisin level and its relationship with metabolic events observe that irisin levels of obese people are higher than average.Citation12,Citation26–28 Lower levels of irisin in type 2 diabetes mellitus, fatty liver and chronic kidney patients were remarkable.Citation9,Citation29–31 Studies were carried out about irisin levels on different tissues following an exercise (kidneys, liver, cardiac muscle, and skeletal muscle). Young rats had no difference in irisin levels after an exercise while older rats had increased irisin levels only in their kidney tissues.Citation11
In light of these data, HPD are getting more and more common as with exercise and reports coming out about how such diets can harm kidneys, we tried to answer multiple questions regarding the subject. These were: (1) to observe the connection between irisin, eNOS, iNOS expression, and localization in rat kidneys; (2) to investigate the effects of HPD taken along exercise on kidney damage; (3) role of irisin, eNOS, and iNOS expression in rats who exercise and fed with HPDs.
Materials and methods
Ethics approval
The present study was conducted in the Local Experimental Animals Research Center after obtaining approval from the Local Experimental Animals Ethics Committee (Decision number: 2015.01.03).
Animal’s studies
Forty adult male Sprague-Dawley rats weighing between 250–320 grams and between 14–16 weeks old were used in our study. All animals were fed daily with tap water and a standard pellet diet for rats under optimum laboratory conditions (temperature: 23 ± 1 °C; humidity: 50–55%; light/dark period: 12 h/12 h). The animals were fasted for 12 h before the experiment. Study was carried out on four different groups: Group 1: normal activity and feed (C group); Group 2: fed normally, but exercised (E group); Group 3: normal activity and HPD (HDP group); Group 4: exercise and high protein feed (exercise-HPD group). Rats that were not in control group were kept on a HPD (45%) for 5 weeks, as on previous studies. E and HPD–E groups were made to exercise on an increasing intensity for 5 weeks.
Experimental diets
Formulation of the experimental diets is presented in . All diets were formulated to meet nutrient requirements of rats following the recommendations of the American Institute of Nutrition (AIN-93M),Citation32 with slight modifications. We selected a protein level of 45% for the HPD groups, following previous studies in which a HPD was compared with NP diets in rats. Feed was prepared with whey protein which is sold by a commercial company.
Table 1. Composition of the experimental diets.
Exercise protocol
Rats were included an exercise program as 5 exercise and 2 rest days per week for 5 weeks, exercising on a treadmill with increasing speed and angle (). Rats were given 10 minutes of adaption work for 2 days, on a treadmill with speed of 1 meter/minute. Rats on exercise group started their program without previous notice on treadmills in a Plexiglas partition with 10 × 12 × 25 cm dimensions. Exercise program started moderately and got harder as experiment progressed.Citation33
Table 2. Exercise program.
At the end of the 5 weeks of study, thiopental sodium (100 mg/kg) anesthesia was applied intraperitoneally to all rats. Heparin was injected (500 U/kg) via femoral vein. Blood and kidney tissue were taken from rats under anesthesia.
Total antioxidant and total oxidant capacity evaluation
Blood samples were centrifuged at 3000 rpm for 15 minutes for total antioxidant (TAC) and oxidant (TOC) capacities measurements. Serum’s TAC and TOC levels were graded with autoanalyzer (Siemens Advia 1800 Chemistry System, Siemens, Tokyo, Japan) using Rel Assay TAC and TOC test kits (Mega Tip San. ve Tic. Ltd., Şti. Gaziantep, Turkey)
Histological and immunohistochemical evaluation
Kidney tissue samples were kept in 10% formalin solution for 24 h at room temperature and then embedded paraffin blocks. They were put through increasing ratio of alcohol series (60%, 70%, 80%, 90%, 99.9%). Tissues were exposed to xylene and paraffinization to increase their transparency and paraffin blocks were made. 4 μ thickness sections stained with hematoxylin & eosin (H&E) and immunohistochemically stained with irisin (Bioss FNDC5 BS-4886-R), iNOS (Spring REF E3744), and eNOS (Neomarkers RB-9279-P) antibodies for general histopathological evaluation. Tissue slides were incubated in 37 °C overnight and 56 °C for 2 h. There times 10 minutes xylene series in 60 °C, 96%, 80%, 70% alcohol series and three times distilled water series were applied. “Citrate buffer 10× pH 8.0” (Code: 15-M820, Lot. 50930) was used for antigen retrieval (heat in 95–100 °C and 20 minutes/cool in room temperature—20 minutes). Phosphate-buffered saline was applied 10 minutes after endogen peroxide blocking with 3% H2O2. Slides were incubated with FNDC5 antibody in room temperature 45 minutes. Later, standard immunoperoxidase staining method steps were applied. Staining was evaluated by pathologists without info about the group’s properties for neutrality. Slides were evaluated with Olympus BX-51 microscope. Axioplan 2 imaging, MC80 DX programs were used to get images from sections. Tissue sections were scored between 0 and 4 with a previously described semi quantitative scale designed to evaluate the degree of tubular necrosisCitation34: 0, normal kidney; 1, minimal necrosis (<5% involvement); 2, mild necrosis (5–25% involvement); 3, moderate necrosis (25–75% involvement); and 4, severe necrosis (>75% involvement). A cast percentage was acquired for each case. All immunopositive cells were scored in a random section of 10 high power fields (400×). Stained cells for each case were scored and put in a percentage. Prevalence of staining graded as 0 (0–5%), 1 (6–24%), 2 (25–49%), 3 (50–74%), and 4 (≥75%). Staining intensity was graded as 0 (negative), 1 (slight), 2 (moderate), and 3 (strong). Two grades were multiplied to get an immunoreactivity score between 0 and 300.
Statistical analysis
SPSS 19 software existing in Trakya University’s Biostatistics Scientific Department was used for statistical analyses. Data are reported as means ± SD. Kruskal–Wallis test was used for comparisons between multiple groups and Mann–Whitney U test was used for comparison of two groups. Significance limit was selected as p < 0.05 for all statistics.
Results
Histopathologic and immunohistochemical evaluation
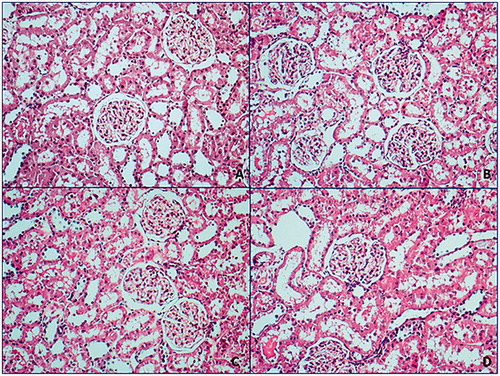
Kidney tissues of all groups were at normal morphology in H&E evaluation and were not detected significant histopathological diversity ().
Figure 1. Normal morphologic properties were followed in all hematoxylin & eosin smears.
A: control group; B: exercise group; C: HPD group; D: exercise-HPD group (100×).
Irisin antibody was shown positive staining on glomerular capillary and peritubular capillary in renal cortex but staining was not observed at tubules (). Staining scores were relatively higher at exercise group yet this difference was not statistically meaningful (p > 0.05) (). eNOS antibody was shown positive staining on distal convoluted tubules while proximal tubules were not stained. Medullary collecting tubules were stained while loop of Henle tubules were not stained. There was not a significant difference between groups regarding eNOS expression (p > 0.05) (). iNOS antibody was shown positive reaction on proximal tubules of cortex while no staining was observed at distal convoluted tubules. Loop of Henle at medulla area was stained too ( ). In both exercise and exercise–HPD groups, iNOS expression were significantly higher than no exercising groups (p < 0.001) ().
Figure 2. Glomerular capillaries at cortex (A) and interstitial area capillaries were observed at vein walls; (B) (red arrow) (FNDC5 antibody, A, B: 400×; C: 25×; D: 200×).
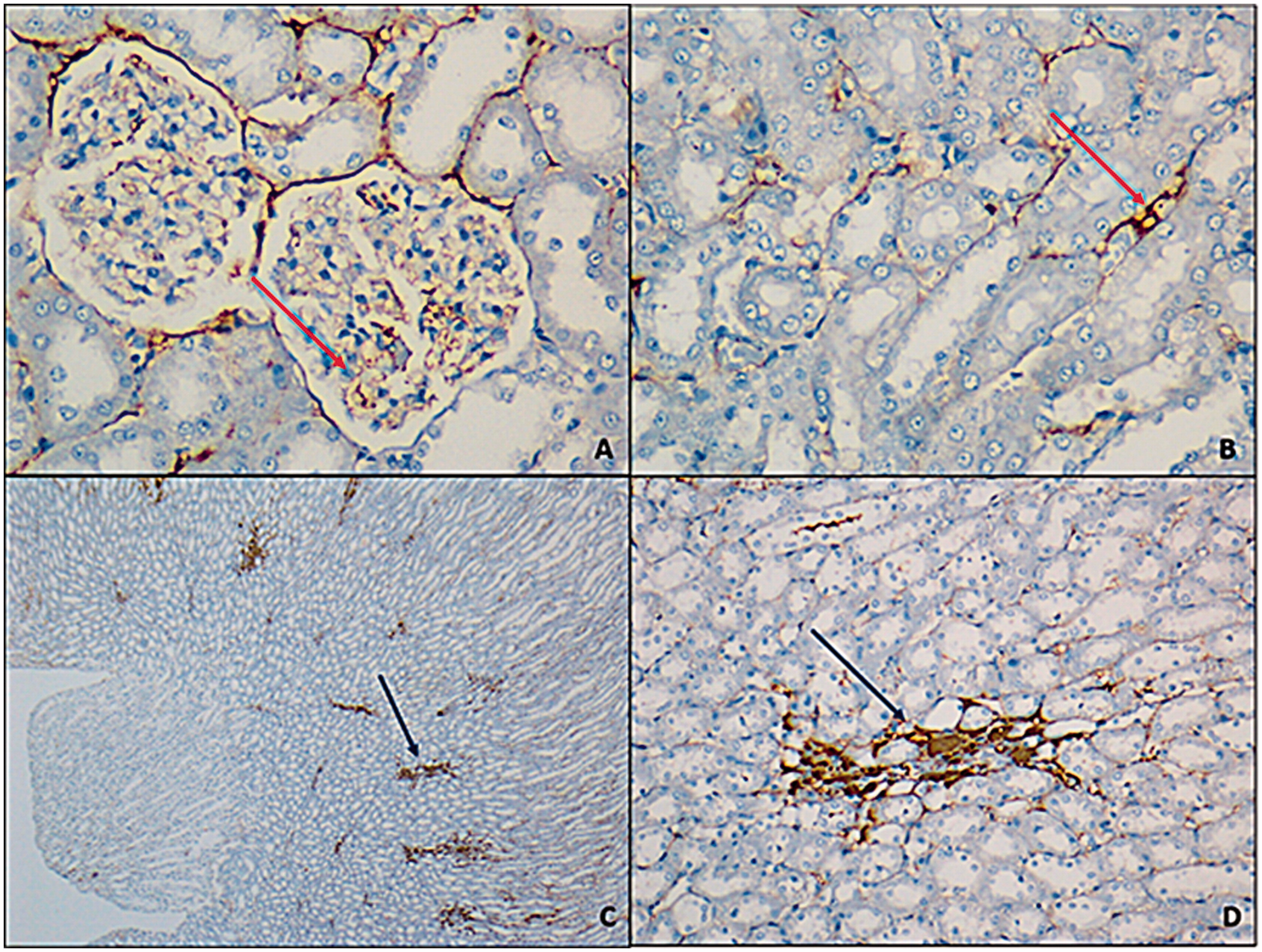
Figure 3. Prevalent and strong smearing with iNOS antibodies were observed at exercise (A), exercise–HPD (B) groups (red arrow). At control group (C) and HPD (D) groups, focal, weak, or moderate smear was observed (200×).
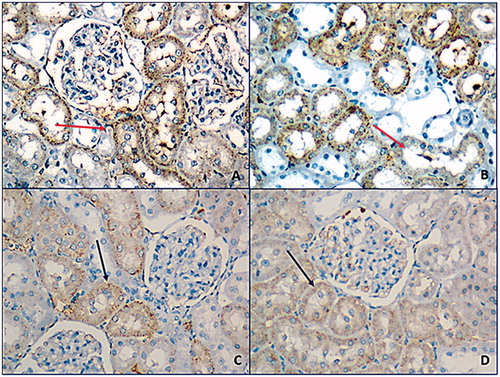
Figure 4. eNOS antibodies smear was at similar levels for all groups (100×). A: control group; B: exercise group; C: HPD group; D: exercise–HPD group.
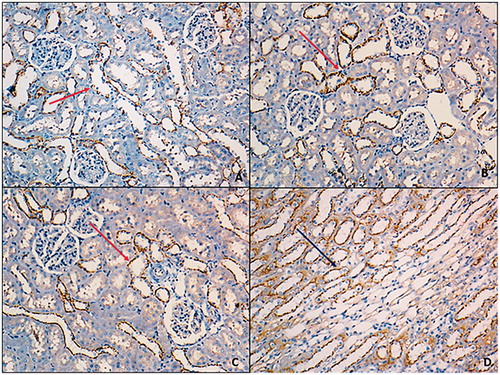
Table 3. Renal tissue eNOS, iNOS, and irisin expression scores.
Antioxidant status evaluation
TAC and TOC levels of serum samples taken after the experiment are displayed at . TAC levels were considerable lower at exercise and exercise–HPD groups in comparison to control groups (p < 0.05, p < 0.001, respectively). HPD group had a higher TAC level in comparison to exercise group (p < 0.05). Exercise group had a higher TAC level than exercise–HPD group (p < 0.001). Exercise–HPD group had a considerably lower TAC levels than HPD group (p < 0.001). No meaningful difference regarding TOC levels of all groups was observed.
Table 4. TAC and TOC level in serum.
Discussion
In this study, we aimed to investigate the effect of exercise and HPD on the release of irisin, eNOS, and iNOS. At the end of our study, no effect of exercise or HPD on irisin expression was observed. Furthermore, we demonstrated that eNOS expression was not related to exercise and HPD. However iNOS score was high in the exercise group and the HPD with exercise group, and low in the HPD group that. Total serum antioxidant value was low in all exercise groups.
Exercise causes a decrease in kidney blood flow, leading to low kidney functioning. Miyauchi et al.Citation18 reported that in exercising rat’s kidney eNOS activity decreases while iNOS activity remains stable, attributing it to decreased kidney functioning under stress. In our study, eNOS level has remained stable in exercise groups. However an increase in iNOS levels has been observed. If the method of Miyauchi et al.Citation18 is examined, the exercise technique is: 5 days a week, for 4 weeks, 10 m/min speed for first 10 minutes and 25 m/min speed for 45 minutes. No floor angle change has been made in this exercise model. In our study, the exercise protocol was: 4 m/min for first 15 minutes with straight floor to 14 m/min for 35 minutes with 3° slope. The differences in exercise speed, duration, and floor angle may be the reason of the difference in iNOS activity levels. Increased cardiac load with floor angle and the stress caused by it may be to blame here.
In studies investigating the relation between irisin levels and exercise there have been conflicting results. These studies report that young individuals exercise does not cause differences in irisin levels.Citation11,Citation12 It is emphasized that irisin levels may be related to age or release may be occurring from the cardiac muscles or another organ and not the skeletal muscles. Furthermore, it may be related to strain or injuries. However, none of these issues have been definitely explained.
In literature, the first study investigating the effect of exercise on FNDC5/irisin expression in the kidney, Aydin et al.Citation11 examined the FNDC5/irisin levels in different tissues (kidney, liver, cardiac muscles, and skeletal muscles). In young rats, FNDC5/irisin levels were stable in all tissues whereas in old rats, FNDC5/irisin levels increased only in the kidney after exercise.Citation11 In our study, the age range of the rats was the same with the young ones in Aydin et al.’s study.Citation11 In our results, lack of changes in FNDC5/irisin levels confirms the data about expression in young rats. Another issue which will be discussed here and provide guidance for future studies is the detection of the location of FNDC5/irisin distribution in the kidney with immunochemical staining. In our study, we observed highly interesting and clearly staining traits in the FNDC5/irisin antibody in kidney tissue. Staining was observed in the cortex, glomerulus capillaries, and peritubular capillaries, but not observed in the tubules. Staining was observed in the loop of Henle’s duct, but not observed in the collecting tubules. No clear information is present in literature concerning the location of FNDC5/irisin expression in the kidney.eNOS immunoreaction has been observed in different areas of the kidney in different studies. eNOS expression has been demonstrated in renal arteries and arterioles, glomerular capillaries, medullary descending vasa recta, IMCD, loop of Henle, and proximal convoluted tubule.Citation35 Equality of cortex and medulla expression has been demonstrated with Western blot analysis. In our study, however eNOS has shown immunoreactivity in the distal convoluted tubule and the medullary collecting canals but not in proximal and distal collecting tubules. This has led us to think that eNOS localization may be different in different models or diseases. In our study, iNOS, eNOS, and irisin antibodies shown very definite and clear staining results. Normally staining localizations of these antibodies must be detected and discussed.
We believe exercise causes stress in the body and this leads to an increase iNOS immunoreactivity. In our study, the decrease in TAC results in exercise groups supports our view. We think that factors such as duration, intensity, and slope must be regulated in a controlled manner.
HPD recommended a dietary program for exercising individuals in daily life. Regulated HPD are reputed to decrease exercise-related stress, as well as help obtain the required energy to feed the tissues with increased metabolic activity.Citation1 In our study, HPD did not have positive effects on TAC decrease in the exercise group. We also see that HPDs while exercising do not assist antioxidant defense. However, our observation of an increase in oxidative score and a decrease in antioxidant levels indicates that precautions to reduce the stress should be taken in those who exercise. Furthermore, we observed that HPDs do not cause histopathological and oxidative damage. This lack of damage may be related to the content of the exercise program.
Our study is not without its limitations. Advanced evaluation methods such as gene expression and Western blot can provide more satisfying results about irisin expression. Furthermore, our tissue damage evaluations with light microscopy must be supported with ultrastructural studies.
In conclusion, in our study, we have obtained some basic ideas about HPD and exercise. (1) Kidneys of rats that exercise have no change in irisin or eNOS expression; however, iNOS exhibits activation in exercising rats. Localization of irisin, eNOS and iNOS is a field open to research. (2) Exercise combined with HPD has not been observed to cause kidney damage histopathologically, however the contents of the diet and exercise protocols are important. (3) HPD combined with exercise does not have a significant effect on rat kidney irisin, eNOS, or iNOS expression.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aparicio VA, Nebot E, Porres JM, et al. Effects of high-whey-protein intake and resistance training on renal, bone and metabolic parameters in rats. Br J Nutr. 2011;105:836–845.
- Jia Y, Hwang SY, House JD, et al. Long-term high intake of whole proteins results in renal damage in pigs. J Nutr. 2010;140:1646–1652.
- Ballal K, Wilson CR, Harmancey R, Taegtmeyer H. Obesogenic high fat western diet induces oxidative stress and apoptosis in rat heart. Mol Cell Biochem. 2010;344:221–230.
- Kartha GK, Moshal KS, Sen U, et al. Renal mitochondrial damage and protein modification in type-2 diabetes. Acta Diabetol. 2008;45:75–81.
- Aydin S. Three new players in energy regulation: Preptin, adropin and irisin. Peptides. 2014;56:94–110.
- Bostrom P, Wu J, Jedrychowski MP, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468.
- Hofmann T, Elbelt U, Stengel A. Irisin as a muscle-derived hormone stimulating thermogenesis – A critical update. Peptides. 2014;54:89–100.
- Kelly DP. Medicine. Irisin, light my fire. Science. 2012;336:42–43.
- Wen MS, Wang CY, Lin SL, Hung KC. Decrease in irisin in patients with chronic kidney disease. PLoS One. 2013;8:e64025
- Aydin S, Aydin S, Kuloglu T, et al. Alterations of irisin concentrations in saliva and serum of obese and normal-weight subjects, before and after 45 min of a Turkish bath or running. Peptides. 2013;50:13–18.
- Aydin S, Kuloglu T, Aydin S, et al. Cardiac, skeletal muscle and serum irisin responses to with or without water exercise in young and old male rats: Cardiac muscle produces more irisin than skeletal muscle. Peptides. 2014;52:68–73.
- Huh JY, Panagiotou G, Mougios V, et al. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism. 2012;61:1725–1738.
- Moreno-Navarrete JM, Ortega F, Serrano M, et al. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J Clin Endocrinol Metab. 2013;98:E769–E778.
- Caglar M, Goksu M, Isenlik BS, et al. Irisin in idiopathic foetal growth restriction. J Endocrinol Invest. 2014;37:619–624.
- Dun SL, Lyu RM, Chen YH, Chang JK, Luo JJ, Dun NJ. Irisin-immunoreactivity in neural and non-neural cells of the rodent. Neuroscience. 2013;240:155–162.
- Chen Q, Xiao DS. Long-term aerobic exercise increases redox-active iron through nitric oxide in rat hippocampus. Nitric Oxide. 2014;36:1–10.
- Eghbalzadeh K, Brixius K, Bloch W, Brinkmann C. Skeletal muscle nitric oxide (NO) synthases and NO-signaling in “diabesity” – What about the relevance of exercise training interventions? Nitric Oxide. 2014;37:28–40.
- Miyauchi T, Maeda S, Iemitsu M, et al. Exercise causes a tissue-specific change of NO production in the kidney and lung. J Appl Physiol. 2003;94:60–68.
- Ramirez DC, Gimenez MS. Induction of redox changes, inducible nitric oxide synthase and cyclooxygenase-2 by chronic cadmium exposure in mouse peritoneal macrophages. Toxicol Lett. 2003;145:121–132.
- Castillo-Quan JI. From white to brown fat through the PGC-1alpha-dependent myokine irisin: Implications for diabetes and obesity. Dis Model Mech. 2012;5:293–295.
- Huh JY, Mougios V, Kabasakalis A, et al. Exercise-induced irisin secretion is independent of age or fitness level and increased irisin may directly modulate muscle metabolism through AMPK activation. J Clin Endocrinol Metab. 2014;99:E2154–E2161.
- Kim HJ, So B, Choi M, Kang D, Song W. Resistance exercise training increases the expression of irisin concomitant with improvement of muscle function in aging mice and humans. Exp Gerontol. 2015;70:11–17.
- Lecker SH, Zavin A, Cao P, et al. Expression of the irisin precursor FNDC5 in skeletal muscle correlates with aerobic exercise performance in patients with heart failure. Circ Heart Fail. 2012;5:812–818.
- Raschke S, Eckel J. Adipo-myokines: Two sides of the same coin–mediators of inflammation and mediators of exercise. Mediators Inflamm. 2013;2013:320724.
- Timmons JA, Baar K, Davidsen PK, Atherton PJ. Is irisin a human exercise gene? Nature. 2012;488:E9E10.
- Crujeiras AB, Pardo M, Arturo RR, et al. Longitudinal variation of circulating irisin after an energy restriction-induced weight loss and following weight regain in obese men and women. Am J Hum Biol. 2014;26:198–207.
- Elbelt U, Hofmann T, Stengel A. Irisin: What promise does it hold? Curr Opin Clin Nutr Metab Care. 2013;16:541–547.
- Joung KE, Park KH, Zaichenko L, et al. Early life adversity is associated with elevated levels of circulating leptin, irisin, and decreased levels of adiponectin in midlife adults. J Clin Endocrinol Metab. 2014;99:E1055–E1060.
- Liu JJ, Liu S, Wong MD, et al. Relationship between circulating irisin, renal function and body composition in type 2 diabetes. J Diabetes Complications. 2014;28:208–213.
- Yang S, Xiao F, Pan L, et al. Association of serum irisin and body composition with chronic kidney disease in obese Chinese adults: A cross-sectional study. BMC Nephrol. 2015;16:16.
- Zhang H-J, Zhang X-F, Ma Z-M, et al. Irisin is inversely associated with intrahepatic triglyceride contents in obese adults. J Hepatol. 2013;59:557–562.
- Reeves PG. Components of the AIN-93 diets as improvements in the AIN-76A diet. J Nutr. 1997;127:S838–S841.
- Casuso RA, Martinez-Amat A, Martinez-Lopez EJ, Camiletti-Moiron D, Porres JM, Aranda P. Ergogenic effects of quercetin supplementation in trained rats. J Int Soc Sports Nutr. 2013;10:3.
- Rabb H, Ramirez G, Saba SR, et al. Renal ischemic-reperfusion injury in L-selectin-deficient mice. Am J Physiol. 1996;271:F408–F413.
- Mount PF, Power DA. Nitric oxide in the kidney: Functions and regulation of synthesis. Acta Physiol. 2006;187:433–446.
