Abstract
Background: The aim of this study was to assess the preventive effect of xuezhikang (XZK) to replace atorvastatin on the contrast media-induced acute kidney injury (CI-AKI).
Methods: The male Sprague–Dawley rats were divided into five groups: group 1 (sham), injected with normal saline; group 2 (XZK), treated with XZK; group 3 contrast media (CM), injected with CM; group 4 (CM + ATO), injected with CM + pretreatment with atorvastatin; group 5 (CM + XZK), injected with CM + pretreatment with XZK. Twenty-four hours after injection with normal saline or CM, the blood sample and the kidneys were collected for the measurement of biochemical parameters, oxidative stress markers, nitric oxide production, inflammatory parameters, as well as renal histopathology and apoptosis detection.
Results: Our results indicated that XZK restored the renal function by reducing serum blood urea nitrogen (BUN) and serum creatinine (Scr), depressing renal malondialdehyde (MDA), increasing renal NO production, decreasing TNF-ɑ and IL-6 expression, attenuating renal pathological changes and inhibiting the apoptosis of renal tubular cells.
Conclusion: XZK’s therapeutic effect is similar, or even better than atorvastatin at the same effectual dose in some parts.
Introduction
Contrast media-induced acute kidney injury (CI-AKI) is a serious hospital-acquired acute renal failure, which is a leading cause of acute kidney injury and is associated with significant mortality and morbidity. It is associated with an absolute increase of 0.5 mg/dl or more, or an increase of 25% or more in serum creatinine (Scr) from baseline value, within 24–72 h following the exposure to contrast media.Citation1,Citation2
Although there are currently no approved pharmacologic agents for the prevention of AKI, several experimental and clinical studies strongly suggested the beneficial effects of statins against contrast media (CM)-induced nephrotoxicity independent of its effects on lipid-lowering, statins possessed pleiotropic effects including improving endothelial function, reducing oxidant stress and direct anti-inflammatory effects.Citation3 However, for the sake of the drug safety, the use of statins in prevention of CI-AKI is still not recommended.
Xuezhikang (XZK), a purified extract of Cholestin, which contains 13 natural occurring statins and the most active one is lovastatin, has been approved by the Food and Drug Administration as a Chinese red-yeast rice dietary supplement. In clinical trials, it was found that treatment with XZK decreased the incidence of myocardial infarction, improved endothelial function, decreased blood lipids, exerted anti-inflammatory actions, and especially, it showed a marked safety in use.Citation4,Citation5 Therefore, the present study was designed to explore the impact and possible mechanisms involved in XZK on CI-AKI in rats model.
Materials and methods
Animals and experimental design
Animals
Male Sprague–Dawley rats (approximately 250–300 g) obtained from Slac Laboratory Animal Co. LTD (Shanghai, China). All animal work was performed according to the Shanghai Jiaotong University School of Medicine guidelines for the ethical care of animals. The protocol was approved by the Committee on the Ethics of Animal Experiments of the Shanghai Jiaotong University School of Medicine.
Treatment groups
The animals were randomly divided into five groups of 10 animals each according to the Scr before any treatment (baseline Scr); G1 (sham), dehydration for three day and furosemide injection 20 min before normal saline injected via tail vein, G2 (XZK (Xuezhikang powder was kindly provided by the WBL Peking University Biotech Co., Luye Pharma Group, Beijing, China) 2400 mg/kg*d, dehydration and XZK administered orally for three days before furosemide injection), G3 (CM, dehydration for three days and furosemide injection 20 min before CM injected), G4 (CM + ATO 20 mg/kg*d, dehydration for three days and furosemide injection 20 min before CM injected), G5 (CM + XZK 2400 mg/kg*d, dehydration for three days and furosemide injection 20 min before CM injected).
Induction of acute kidney injury by contrast media
We used a novel-established model by ourselves of CI-AKI in rats.Citation6 Furosemide (Harvest Pharmaceutical Co., Shanghai, China) was injected at 10 ml/kg intramuscularly. Omnipaque (350 mg I/ml, GE Healthcare, Shanghai, China), a nonionic low-osmolar contrast media, was injected at 20 ml/kg via tail vein administration over the course no less than 5 min. About 24 h after contrast media administration, final blood samples were collected in centrifuge tubes to impair coagulation from the abdominal aorta in rats. After sacrifice, both kidneys were immediately harvested; one kidney was embedded in paraffin for histological studies, the other one was stored in liquid nitrogen for biochemical analysis.
Renal function test
After 15 min of centrifugation at 4000 g, the upper layer of serum was collected to measure Scr and blood urea nitrogen (BUN) using automatic biochemistry analyzer at the Clinical Laboratory of Renji Hospital, School of Medicine, Shanghai Jiaotong University.
Determination of oxidative stress
Levels of malondialdehyde (MDA) were measured using a commercial kit (Beyotime Biotechnology, Shanghai, China). Sample absorbance was assayed at 532 nm using a microplate spectrophotometer and calculated by the absorbance of a standard.
Determination of glutathione
The glutathione (GSH) in the kidney tissue was measured enzymatically according to the manufacture’s instruction (Beyotime Biotechnology, Shanghai, China). The kidney tissue was homogenized with 1 ml of protein removal agent supplied by the kit in a ratio of 1:10 (wt/vol). The homogenate was centrifuged at 10,000 g for 10 min at 4 °C and the supernatant was used for GSH assay. After 25 min incubation, the absorbance was monitored at 412 nm using a microplate spectrophotometer and the amount of GSH was calculated according to the standard curve.
Determination of nitric oxide
The levels of nitric oxide (NO) in kidney tissues were determined indirectly using the Griess reaction for simultaneous evaluation of nitrite and nitrate concentrations as described by the instruction by the manufacturer (Cayman Chemical, Ann Arbor, MI). Kidney tissue was homogenized in 0.1M sodium phosphate buffer pH 7.4 in 1:10 (wt/vol) and centrifuged at 12,000 g for 10 min. Calculation of total nitric oxide was based on nitrite and nitrate values.
Determination of protein
Concentrations of protein were determined by Bradford protein assay reagent (Beyotime Biotechnology, Shanghai, China).
Western blotting
The renal tissue was homogenized in lysis buffer and centrifuged at 15,000 rpm for 20 min at 4 °C to obtain the supernatant. Proteins were loaded onto 10% SDS/PAGE. The gels were transferred to a nitrocellulose membrane and reacted with followed antibodies; Bax and Bcl-2 (Cell Signaling Technology, Danvers, MA). The ECL Western Blotting System was obtained from Beyotime Biotechnology, Shanghai. The protein expression comparison was analyzed by the gray scale image by utilizing software Image J (Bethesda, MD).
TUNEL assay
Kidney samples cut to 5 μm sections were detected by in situ cell death detection kit (Roche, Mannheim, Germany) and observed under a fluorescent microscope. TUNEL-positive cells were then counted by densitometry for integrate doptical density (IOD) using Image-ProPlus-6.0 software (Bethesda, MD) in a blinded manner.
Histological examination
The kidney was fixed immediately in 10% formalin after removal by embedding in paraffin, was sectioned at 3 μm thickness and the sections were stained with hematoxylin and eosin. The extents of tubular injury, vacuolation, necrosis, medullary congestion and proteinaceous casts were evaluated semi quantitatively with a slight microscope as suggested by Yamasowa et al.Citation7
Statistical analysis
Data are expressed as mean ± SEM. Analyses were performed with SPSS (Armonk, NY). Group comparisons were evaluated by one-way-ANOVA followed by Bonferroni’s test. Within-subject comparisons of continuous variables were carried out by a paired t-test. p Values of <0.05 was considered statistically significant.
Results
Biochemical parameters
The biochemical parameters were presented in . Compared with the rats in sham group and XZK group, contrast media significantly increased BUN (p < 0.05) and Scr (p < 0.01), but Scr was notably reduced in group CM with pretreatment of atorvastatin or XZK to the similar level of sham group and XZK group (p < 0.05, vs. group CM) ().
Table 1. Renal functional parameters in study groups.
Oxidative stress markers
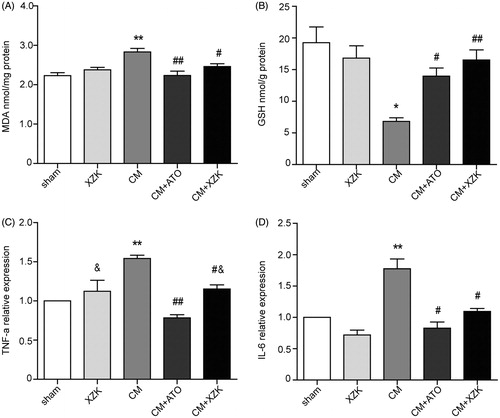
Among the five experimental groups, kidney MDA level in group CM was the highest (2.83 ± 0.09 nmol/mg protein), and its GSH level was the lowest (6.81 ± 0.11 nmol/g protein). There was significant statistical difference of MDA when compared with the group sham, XZK, CM + ATO (p < 0.01) and group CM + XZK (p < 0.05) (). Similarly, there was significant statistical difference of GSH in group CM when compared with the group sham, XZK, CM + XZK (p < 0.01) and group CM + ATO (p < 0.05) ().
Figure 1. (A) MDA concentration of study groups. (B) Glutathione concentration of study groups. (C) TNF-α relative expression in study groups. (D) IL-6 relative expression in study groups. *p < 0.05, **p < 0.01 versus group sham and XZK; #p < 0.05, ##p < 0.01 versus group CM; &p < 0.05, versus group CM + ATO. n = 10. Values are mean ± SEM.
Inflammatory parameters
In group CM, both TNF-ɑ and IL-6 in kidney were notably higher than group sham and group XZK (p < 0.01). Meanwhile, TNF-ɑ and IL-6 of group CM were also much higher than group CM + ATO (p < 0.01) and CM + XZK (p < 0.05). Moreover, there existed difference between TNF-ɑ of group CM + ATO and group XZK and CM + XZK (p < 0.05) ().
Renal nitric oxide production
From , kidney total NO (nitrite/nitrate) levels of group CM was lower than group sham and XZK (p < 0.05). On the contrary, the total NO (nitrite/nitrate) level of kidney in group CM pretreated with ATO or XZK was higher than group CM (p < 0.05 and p < 0.01, respectively).
Table 2. Nitric oxide and nitrite/nitrate levels (nmol/mg protein) in study groups (mean ± SEM).
Histological changes
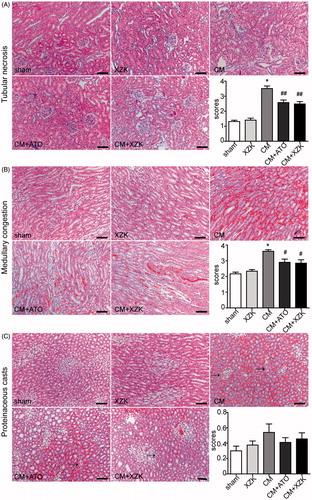
All rats in group CM, group CM + ATO and CM + XZK developed obvious tubular necrosis and medullary congestion in the renal medullary region. Nevertheless, it exhibited much fewer tubular necrosis and medullary congestion with lower medullary damage scores in the two CM groups with pretreatment compared to the group CM (). Meanwhile, the degree of proteinaceous casts was so mild that the scores of these 5 groups were all low and without difference among them ().
Figure 2. Histological changes of renal tissue in study groups (H&E staining, scale bar, 100 μm). (A) Tubular necrosis. Tubular necrosis was graded as follows: no damage (0), mild (1, patchy isolated damage), moderate (2, damage less than 25%), severe (3, damage between 25 and 50%), and very severe (4, more than 50% damage). (B) Medullary congestion, The degree of medullary congestion was defined as follows: no congestion (0), mild (1, vascular congestion with identification of erythrocytes by ×400 magnification), moderate (2, vascular congestion with identification of erythrocytes by ×200 magnification), severe (3, vascular congestion with identification of erythrocytes by ×100 magnification) and very severe (4, vascular congestion with identification of erythrocytes by ×40 magnification). (C) Proteinaceous casts. Proteinaceous casts were graded as follows: no damage (0), mild (1, unicellular, patchy isolated damage), moderate (2, damage less than 25%), severe (3, damage between 25 and 50%), and very severe (4, more than 50% damage). *p < 0.01 versus group sham and group XZK; #p < 0.05, ##p < 0.01 versus group CM. n = 10. Values are mean ± SEM.
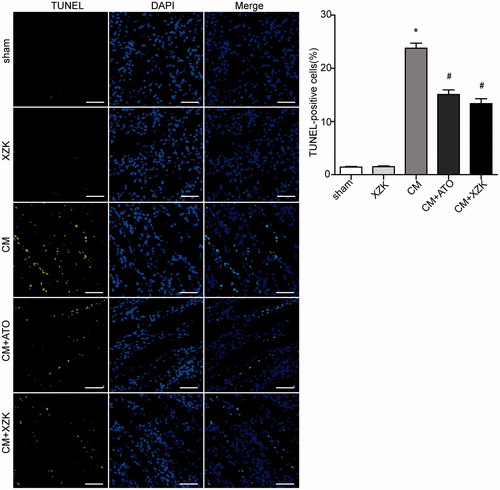
TUNEL assay
Consistent with the results of histology, the percentage of apoptotic cells was significantly higher in the groups administrated with CM (group CM, group CM + ATO and CM + XZK) than the group sham and group XZK. However, the percentage of TUNEL-positive renal tubular cells was lower in the group CM + ATO and CM + XZK than group CM (p< 0.01) (.
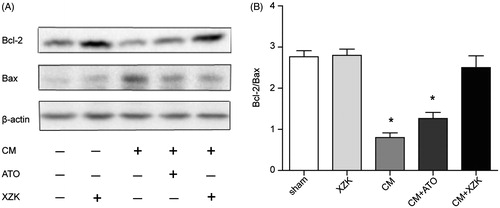
Expression of Bax and Bcl-2
The expression of anti-apoptotic (Bcl-2) and pro-apoptotic (Bax) markers were detected by Western blotting. The group CM showed a down-regulated expression of Bcl-2 and an up-regulated expression of Bax when compared with the group sham and group XZK, leading to a decrease in Bcl-2/Bax ratio. On the contrary, group CM + ATO and CM + XZK augmented the expression of Bcl-2 and inhibited the expression of Bax. Accordingly, the ratio of Bcl-2/Bax was appreciably higher in group CM + XZK, compared with group CM and CM + ATO (p < 0.01) (.
Discussion
Although, several recent meta-analysis and clinical trials concluded a potential benefit for use of high dose statins prior to angiography to prevent the incidence of CI-AKI,Citation8–10 clinically, concerning the safety of high-dose statins in patients at high risk where they may have skeletal muscle or liver adverse effects, it is not recommended by the latest management of AKI guideline yet. Thus, to find a safe yet effective therapeutic agent for use to replace statins for the treatment of CI-AKI became a big challenge for doctors, especially when the patient population is aging and diabetes and chronic kidney diseases are becoming common.
XZK has been demonstrated to be a cautious treatment of the secondary prevention in older individuals for its efficiency in the management of hyperlipidemia and coronary heart disease (CHD) as well as its low incidence of negative effect.Citation5,Citation11 In the present study, we further verified its security in use and demonstrated that pretreatment with XZK in CI-AKI significantly decreased oxidative damage, inflammatory responses, attenuated renal tubular cell apoptosis and restored the renal function, resulting in ameliorated renal function.
The central role in the pathophysiology of CI-AKI is a significant reduction in functional nephrons.Citation1,Citation2 After a transient phase of vasodilation, the release of adenosine, endothelin, inhibition of nitric oxide-mediated vasodilation and changes in calcium concentration in smooth muscle triggered by iodinated contrast caused sustained reduction in renal blood flow. It contributed to the ischemia of the outer regions of the medulla, further increasing tubular cell injury. Meanwhile, iodinated contrast accumulated in the renal tubules and collecting ducts leaded to direct cellular injury. Except for these two mechanisms, it is believed that oxidative stress and inflammation may play an indirect role in other organ injury processes.Citation12–14 The former one altered oxygen balance leading to medullary hypoxic injury manifesting as cell apoptosis and necrosis.Citation12,Citation13 The later one initiated the tissue damage through inflammatory factors,Citation14–16 i.e. TNF-ɑ and IL-6. However, the detailed mechanism of CM-induced renal impairment at cellular level is not fully understood yet.
One of the protective effects of XZK against CM can be attributed to its anti-oxidant property. Membrane lipid-peroxidation in terms of MDA and intracellular GSH is considered to be a reliable marker of oxidative stress. We observed a significant increase in MDA and a decrease in GSH in kidneys of rats exposed to CM, indicating a state of oxidative stress. Treatment of rats with atorvastatin or XZK reversed CM induced oxidative stress, and there was no difference between them. In addition, biochemical analysis of kidney showed a significant decrease in total NO (nitrate/nitrite) levels in our CI-AKI rat model, because it was reported that nonionic CM-induced endothelial dysfunction decreased eNOS expression and increased plasma endothelin-1, which have been implicated in aggregating platelet, enhancing vasoconstriction and ischemic tubular injury, resulting in cell detachment, apoptosis or necrosis and kidney injury eventually.Citation17 However, both atorvastatin and XZK exerted a positive influence on NO resulting in improved microcirculation. Interestingly, NO level of CM group pretreated with XZK was higher than atorvastatin at the similar dose in our study.
Additionally, consistent with the results of previous work provided, evidence that XZK was able to repair the kidney damage by down-regulating the expression levels of inflammatory transcription factors caused by hyperlipidemia,Citation18 both atorvastatin and XZK pretreatment attenuated the renal expression levels of TNF-α and IL-6, compared with the CM group and atorvastatin revealed a bit more significant anti-inflammatory effect. High expression of TNF-α and IL-6 were impaired to tissues, and they were associated with CIN risk and poor long-term renal outcome after PCI.Citation19–21 It is because of TNF-ɑ which was proven to play a central role in the activation of the inflammatory cytokine responseCitation19,Citation20 and IL-6 is associated with response to pro-inflammatory signals involved in tissue injury and organ failure.Citation21,Citation22.
On the other hand, renal pathological changes caused by contrast media including necrosis of renal tubular epithelial cells, proteinaceous casts in renal tubules and medullary congestion, were improved by atorvastatin and XZK, without difference between them. In addition, the tubular cell apoptosis was lessened, which is confirmed not only by HE staining but also by TUNEL assay in groups pretreated with atorvastatin or XZK. In agreement with these results, compared with the CM group, the group pretreated with atorvastatin or XZK revealed an up-regulation of Bcl-2 and a down-regulation of Bax with a higher Bcl-2/Bax ratio. Due to the ratio of intracellular Bcl-2 to Bax being more significant than their individual absoluteCitation23–25 values, which might play a pivotal role in the apoptosis process, XZK seemed more anti-apoptotic.
Although the main effective ingredient of XZK was believed to be lovastatin, it is suggested by the present study that XZK (2400 mg/kg*d) exhibited a more outstanding effect than that of the same dose of atorvastatin (20 mg/kg*d) on high NO production and Bcl-2/Bax ratio in CI-AKI, except for the similar therapeutic effect on reducing BUN, Scr, renal levels of MDA, TNF-a and IL-6 expression as well as greatly attenuating the morphological changes. These findings are likely to attribute to the character of XZK as a polypill,Citation26,Citation27 containing numerous natural statins as well as unsaturated fatty acids, flavonoids, ergosterol, amino acids, alkaloids, trace elements of plant sterols and a number of other biologically active substances.
In conclusion, this study sheds light on XZK and states that it is a safe and effective therapeutic agent capable of reducing kidney injury caused by contrast media.
Disclosure statement
The authors declare no conflicts of interest in preparing this article.
Funding
This work was supported by the National Natural Science Foundation [Grant Numbers 81330006, 91539106, 81370399, 81500266] and the Shanghai Municipal Commission of Health and Family Planning [Grant Numbers 20154Y0036]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Peter A. McCullough contrast-induced acute kidney injury. J Am College Cardiol. 2008;51:1419–1428.
- Stacul F, van der Molen AJ, Reimer P, et al. Contrast induced nephropathy: Updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol. 2011;2:2527–2541.
- Gazzerro P, Proto MC, Gangemi G, et al. Pharmacological actions of statins: A critical appraisal in the management of cancer. Pharmacol Rev. 2012;64:102–146.
- Zhao SP, Liu L, Cheng YC, Li YL. Effect of xuezhikang, a Cholestin extract, on reflecting postprandial triglyceridemia after a high-fat meal in patients with coronary heart disease. Atherosclerosis. 2003;168:375–380.
- Liu L, Zhao SP, Cheng YC, Li YL. Xuezhikang decreases serum lipoprotein(a) and C-reactive protein concentrations in patients with coronary heart disease. Clin Chem. 2003;49:1347–1352.
- Sun SQ, Zhang T, Nie P, et al. A novel rat model of contrast-induced acute kidney injury. Int J Cardiol. 2014;172:e48–e50.
- Yamasowa H, Shimizu S, Inoue T, et al. Endothelial nitric oxide contributes to the renal protective effects of ischemic preconditioning. J Pharmacol Exp Ther. 2005;312:153–159.
- Marenzi G, Cosentino N, Werba JP, Tedesco CC, Veglia F, Bartorelli AL. A meta-analysis of randomized controlled trials on statins for the prevention of contrast-induced acute kidney injury in patients with and without acute coronary syndromes. Int J Cardiol. 2015;183:47–53.
- Hoshi T, Sato A, Kakefuda Y, et al. Preventive effect of statin pretreatment on contrast-induced acute kidney injury in patients undergoing coronary angioplasty: Propensity score analysis from a multicenter registry. Int J Cardiol. 2014;171:243–249.
- Han Y, Zhu G, Han L, et al. Short-term rosuvastatin therapy for prevention of contrast-induced acute kidney injury in patients with diabetes and chronic kidney disease. J Am Coll Cardiol. 2014;63:62–70.
- Zhao SP, Liu L, Cheng YC, et al. Xuezhikang, an extract of cholestin, protects endothelial function through antiinflammatory and lipid-lowering mechanisms in patients with coronary heart disease. Circulation. 2004;110:915–920.
- Quintavalle C, Brenca M, De Micco F, et al. In vivo and in vitro assessment of pathways involved in contrast media-induced renal cells apoptosis. Cell Death Dis. 2011;2:e155.
- Guerchicoff A, Stone GW, Mehran R, et al. Analysis of biomarkers for risk of acute kidney injury after primary angioplasty for acute ST-segment elevation myocardial infarction: Results of the HORIZONS-AMI trial. Catheter Cardiovasc Interv. 2015;85:335–342.
- Toso A, Leoncini M, Maioli M, et al. Relationship between inflammation and benefits of early high-dose rosuvastatin on contrast-induced nephropathy in patients with acute coronary syndrome: The pathophysiological link in the PRATO-ACS study (Protective Effect of Rosuvastatin and Antiplatelet Therapy on Contrast-Induced Nephropathy and Myocardial Damage in Patients With Acute Coronary Syndrome Undergoing Coronary Intervention). JACC Cardiovasc Interv. 2014;7:1421–1429.
- Gao F, Zhou YJ, Zhu X, Wang ZJ, Yang SW, Shen H. C-reactive protein and the risk of contrast-induced acute kidney injury in patients undergoing percutaneous coronary intervention. Am J Nephrol. 2011;34:203–210.
- Fan XF, Deng YQ, Ye L, et al. Effect of Xuezhikang capsule on serum tumor necrosis factor-alpha and interleukin-6 in patients with nonalcoholic fatty liver disease and hyperlipidemia. Chin J Integr Med. 2010;16:119–123.
- Zhu XY, Li P, Yang YB, Liu ML. Xuezhikang, extract of red yeast rice, improved abnormal hemorheology, suppressed caveolin-1 and increased eNOS expression in atherosclerotic rats. PLoS One. 2013;8:e62731.
- Ding M, Si DY, Zhang WQ, Feng ZH, He M, Yang P. Red yeast rice repairs kidney damage and reduces inflammatory transcription factors in rat models of hyperlipidemia. Exp Ther Med. 2014;8:1737–1744.
- Chang CF, Lu TM, Yang WC, Lin SJ, Lin CC, Chung MY. Gene polymorphisms of interleukin-10 and tumor necrosis factor-α are associated with contrast-induced nephropathy. Am J Nephrol. 2013;37:110–117.
- Machado RA, Constantino Lde S, Tomasi CD, Rojas HA, Vuolo FS, Vitto MF. Sodium butyrate decreases the activation of NF-κB reducing inflammation and oxidative damage in the kidney of rats subjected to contrast-induced nephropathy. Nephrol Dial Transplant. 2012;27:3136–3140.
- Hudzik B, Szkodzinski J, Danikiewicz A, Romanowski W, Lekston A, Polonski L. Serum interleukin-6 concentration predicts contrast-induced nephropathy in patients undergoing percutaneous coronary intervention. Eur Cytokine Netw. 2010;21:129–135.
- Zhang ZX, Huang X, Jiang J, et al. (2014) Natural killer cells play a critical role in cardiac allograft vasculopathy in an interleukin-6-dependent manner. Transplantation. 2014;98:1029–1039.
- Kaszuba-Zwoinska J, Ziomber A, Gil K, Bugajski A, Zaraska W, Thor P. Pulsating electromagnetic field induces apoptosis of rat’s bowel Cajal’s cells. Folia Med Cracov. 2005;46:87–85.
- An S, Hishikawa Y, Liu J, Koji T. Lung injury after ischemia-reperfusion of small intestine in rats involves apoptosis of type II alveolar epithelial cells mediated by TNF-alpha and activation of Bid pathway. Apoptosis. 2007;12:1989–2001.
- Abdelkader NF, Safar MM, Salem HA. Ursodeoxycholic acid ameliorates apoptotic cascade in the rotenone model of Parkinson’s disease: Modulation of mitochondrial perturbations. Mol Neurobiol. 2016;53:810–817.
- Lu Z, Kou W, Du B, et al. Effect of Xuezhikang, an extract from red yeast Chinese rice, on coronary events in a Chinese population with previous myocardial infarction. Am J Cardiol. 2008;101:1689–1693.
- Li JJ, Hu SS, Fang CH, et al. Effects of Xuezhikang, an extract of cholestin, on lipid profile and C-reactive protein: A short-term time course study in patients with stable angina. Clin Chim Acta. 2005;352:217–224.