Abstract
Purpose: Oxidative stress due to hyperglycemia is a major cause of diabetes complications. The aim of this study was to evaluate the effects of pomegranate seed oil (PSO) on serum biochemical parameters, cardiomyopathy and nephropathy induced by diabetes mellitus.
Method: W/A adult rats were divided into four groups (12 each): group 1, received saline (1 mL/kg), group 2, received streptozotocin (STZ, 65 mg/kg, a single dose as i.p.), groups 3 and 4, received STZ + PSO (0.4 and 0.8 mL/kg, daily by gavage, respectively). After three weeks, six rats of each group and one week later the remaining animals were anesthetized, blood samples were taken for measuring serum biochemical parameters. Sections of heart and kidneys were used for histopathological studies and the remaining tissues were homogenized for measuring malondialdehyde (MDA) and total sulfhydryl groups.
Results: Significant elevation of serum creatinine and urea, LDL, triglyceride, glucose levels as well as urine markers, MDA levels in tissue homogenates and a significant decrease in total thiol content and serum HDL were observed in STZ-treated group as compared with control group. PSO treatment resulted in a significant decrease in tissue MDA content, serum creatinine and urea levels as well as urine markers as compared with STZ-treated group. Lipid profile was ameliorated with PSO treatment. PSO also significantly reversed STZ-induced depletion in thiol content and histological abnormality. Effect of PSO was more specific at 28th than 21th days of study.
Conclusion: The results showed that PSO has a protective effect against diabetes complications in rats.
Introduction
Diabetes mellitus (DM) is a chronic metabolic disease portrayed by serum glucose elevation and higher glycated hemoglobin (HbA1C) as well as protein, resulting from defects in action or secretion of insulin, or both.Citation1 Nowadays, about 285 million people are affected with DM in the world and it is estimated that by the year 2030, 439 million people are relied upon to experience from the disease along with its serious complications.Citation2 Chronic hyperglycemia is associated with reactive oxygen species (ROS) production and induced long-term pathological damage, dysfunction, and failure of different organs, especially the kidneys, eyes, nerves, blood vessels, and heart due to increasing oxidative stress. Also biochemical parameters are severely affected by oxidative stress, which result in damages to DNA and cellular proteins.Citation3,Citation4
Higher mortality rate in diabetic patients is related to the complications including cardiovascular diseases (CVDs) and diabetic nephropathy. Dyslipidemia is an important risk factor for CVDs.Citation5 Enhanced oxidative stress due to chronic hyperglycemia is a fundamental reason for next events including endothelial dysfunction and decreasing synthesis and releasing of nitric oxide by endothelial cells that tends to microvascular complications. Adverse effects of dyslipidemia due to insulin resistance including lipoprotein oxidation, production of triglyceride-rich VLDL particles (fasting hypertriglyceridemia) and transient hypertriglyceridemia and changing in lipoprotein metabolism are the other reasons of diabetes complications.Citation5–7
Pomegranate, Punica granatum L., as a fruit native to Middle East, has therapeutic effects including antioxidant,Citation8 anti-inflammatory,Citation9 anti-cancer and anti-microbial.Citation10 Pomegranate seed oil (PSO) made of 12–20% of total seed weight is a rich source of conjugated octadecadienoic fatty acids.Citation11 Antioxidant and anti-inflammatory activities are the main features of PSO due to its content of conjugated linolenic acids especially punicic acid.Citation12 Nephroprotection,Citation13,Citation14 anti-osteoporosis,Citation15 beneficial effect on serum lipid profile,Citation16 neuroprotective effect of PSO,Citation17 reducing peripheral insulin resistanceCitation11 and hepatoprotectionCitation18 are the other beneficial effects of PSO.
Oxidative stress has a role in the etiology of DM complications; therefore, progression of therapeutic factors with antioxidant effects is considered.Citation19 Medicinal plants as an alternative source of therapeutic compounds are interested to the researchers for use as complementary medicine.Citation20 In this study, protective effects of PSO on oxidative stress markers, serum biochemical parameters and pathological findings in kidney and heart tissues of STZ-induced diabetic rats is investigated
Material and methods
Animals
Forty-eight adult male Wistar rats (Animal House, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran), weighing 235–275 g, were used for all experiments. These animals were housed in a clean rodent room under a 12:12-h light–dark cycle, with ad libitum access to food and water and maintained at a temperature of 24 ± 1 °C. The rats were fed standard diet having the following composition: fat, 2–3%; starch, 55%; protein, 20–21%; crude fiber, 6%; NaCl, 5%; and trace elements and amino acids. All animal procedures were approved by the Mashhad University Ethics Committee and were in compliance with National Laws and with National Institutes of Health guidelines for the use and care of laboratory animals.
Chemicals
DTNB (2,20-dinitro-5,50-dithiodibenzoic acid), n-butanol, Na2EDTA (ethylenediaminetetraacetic acid disodium salt), TBA (2-thiobarbituric acid), Trizma base [Tris(hydroxymethyl)aminomethane], KCl, HCl, phosphoric acid ((1%), TCA (trichloroacetic acid) and methanol were purchased from Merck (Darmstadt, Germany). PSO d = 0.81 g/mL at 25 °C was purchased from Sarouneh Institute. (Uromeya, Iran). STZ (streptozotocin), ketamine, and xylazine were obtained from Sigma Aldrich (St. Louis, MO).
Experimental design
Experimental design and induction of STZ model of diabetes
After acclimatization, animals were randomly divided into four groups (12 each) and individually put in the metabolic cages. Group 1, control group, received saline (1 mL/kg) orally, once a day. Groups 2, 3 and 4 were injected with a single dose of STZ (65 mg/kg), dissolved in 0.1 M citrate buffer (pH: 4.5) and used by single i.p. injection 3 days before PSO treatment to induce DM. The development of DM in rats was confirmed by blood glucose evaluation after 48 h. After overnight fasting, blood samples were taken from tail-vein, the animals with blood glucose higher than 250 mg/dL, which was detected by a strip-operated blood glucose meter (Accu-Check Compact Plus, Roche Diagnostics, Germany) were considered as diabetic and were selected for further studies. Groups 3 and 4 were treated daily with PSO, 0.4 and 0.8 mg/kg orally, respectively. The doses of PSO and STZ were selected based on the previous studies.Citation14,Citation20 All procedures were performed between 10 and 12 am. Twenty-four-hour urine samples were collected from six animals of each group for measuring protein and glucose concentrations, one day before surgery at the 20th day of onset of protocol. After overnight fasting, animals were immediately anesthetized by injection of ketamine (60 mg/kg) and xylazine (10 mg/kg). After opening the chest cavity, blood samples were collected by cardiac puncture to measuring serum biochemical parameters. The serum was separated after centrifugation at 1800 g for 10 min and stored at −20 °C. Kidneys and heart were quickly excised for histopathological studies, and biochemical parameters were measured (malondialdehyde and total thiol content), and stored at −70 °C. One week later, the six remaining animals in each group were killed and the samples were taken as described.
Homogenization protocol
The left kidney and heart were scissors minced, washed with normal saline (0.9%) and homogenized in ice shower containing 4 mL of 0.2 M phosphate buffer (pH 7.4). Homogenates were centrifuged at 3000 g for 15 min at 4 °C to remove tissue remnants. Supernatant was restored at −70 °C until measuring the parameters.
Biochemical methods
Lipid profile, glucose and C-reactive protein assay method
The level of triacylglycerol was determined, using enzymatic kits (Ziest Chem Diagnostic kits, Iran) with utilizing glycerol as a standard. Also total cholesterol, HDL and LDL levels were determined based on enzymatic methods by diagnostic kits (Ziest Chem, Iran), with utilizing cholesterol as a standard. Serum glucose and C-reactive protein (CRP) were determined using the Hitachi 7070 automatic biochemical analyzer (Hitachi Ltd., Japan).
Creatine kinase and lactate dehydrogenase
Serum creatine kinase (CK) and lactate dehydrogenase (LDH) activities were measured spectrophotometrically (UV-160, Shimadzu) in a blinded manner by an automatic biochemistry analyzer using a commercially available kit. The absorbance of the solution for LDH was detected at 492 nm and for CK at 340 nm.
Urea and creatinine measurement
Urea concentration was determined colorimetrically with urea kit (Man Lab Company, Tehran, Iran). Creatinine concentration was measured by Jaffe’s method.Citation21
Urine glucose and protein measurement
Urine protein concentration was measured by the turbidimetric method and glucose concentration was evaluated by the enzymatic assay (glucose oxidase).Citation22,Citation23
Measuring MDA content in tissue homogenates
The lipid peroxidation level of the kidney and heart tissues was measured by MDA, which is the end product of lipid peroxidation and reacts with TBA as a thiobarbituric acid reactive substance (TBARS) to produce a red-colored complex which has peak absorbance at 532 nm.Citation24
Briefly, 3 mL phosphoric acid (1%) and 1 mL TBA (0.6%) were added to 0.5 mL of homogenate in a centrifuge tube and the mixture was heated for 45 min in a boiling water bath. After cooling, 4 mL of n-butanol was added to the mixture, vortexed for 1 min. After centrifugation (900 g, 10 min), TBARS was determined by the solution absorbance at 532 nm.
Measuring of total thiol groups
Total SH groups were measured using DTNB as the reagent. One milliliter of Tris–EDTA buffer (pH 8.6) was added to 50 mL tissue homogenate in 2 mL cuvettes and absorbance was read at 412 nm against Tris–EDTA buffer alone (A1). Then, 20 mL DTNB reagent (10 mM in methanol) was added to the mixture, and after 15 min the sample absorbance was read again (A2). The absorbance of DTNB reagent was also read as a blank (B). Total thiol concentration (mm) was calculated from the following equation:Citation25
Histological method
The right kidney and a transverse section of septum of heart and ascending aorta were washed by saline (0.9%), then fixed in formalin (10%), processed, and embedded in paraffin, sectioned and stained by hematoxylin and eosin (H&E) for histopathological assay.
Statistical analysis
Data were expressed as mean ± SEM. Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by the Tukey–Kramer post hoc test for multiple comparisons. p values <0.05 were considered as statistically significant.
Results
Biochemical parameters
Animal’s body weight
Initial and final body weights of rats are shown in . A significant decrease in the final body weights was observed in all groups (p < 0.01, in group II and p < 0.05, in groups III and IV), compared to control group. Diabetic animals showed a significant increase in the heart/body weight ratio level compared to control group after 4 weeks of study. PSO treated groups showed no changes in the heart/body weight ratio compared with STZ-treated group.
Table 1. Initial and the final body weight and the weight of heart in all groups.
Lipid profile, serum glucose and CRP values
The alterations of lipid profile, serum glucose, CRP and number of mortality in each group are shown in . STZ-treated animals resulted significant elevation in serum glucose (p < 0.001), serum triglyceride (p < 0.05, after 3 weeks), serum LDL (p < 0.01, after 3 weeks). PSO-treated groups resulted in a significant reduction in the serum glucose level compared to STZ-treated group (371.3 ± 31.5 vs. 468.2 ± 29.5 mg/dL after 3 weeks and 366.2 ± 27.5 vs. 466.2 ± 32.5 mg/dL after four weeks, p < 0.01 for group 3 and 315.6 ± 30.9 vs. 468.2 ± 29.5 mg/dL after 3 weeks and 308.2 ± 35.4 vs. 466.2 ± 32.5 mg/dL after 4 weeks, p < 0.01for group 4, respectively). There was no significant difference in total cholesterol level between PSO-treated and STZ-treated groups except in group III after 4 weeks (p < 0.05).
Table 2. The serum level of CRP, glucose, lipid profile, and number of mortality in rats.
Serum LDL level was significantly decreased in all treated groups (except in group IV after 4 weeks) compared to STZ-treated group (p < 0.01 in group III and p < 0.05 in group IV). Serum HDL level was also significantly increased by treatment with PSO only in group IV (p < 0.05). Serum TG level was significantly decreased in all PSO-treated groups (p < 0.01). CRP level was positive only in STZ-treated group after 4 weeks and in this group there was one mortality.
CK and LDH activity
The serum CK and LDH activity are also reported in . As shown, there were a significant increase in LDH activity in STZ-treated group compared to control group (p < 0.001) and a significant reduction in PSO-treated groups compared to STZ-treated groups (p < 0.05 in group III after 4 weeks and p < 0.01 in group IV). CK activity was significantly increased in STZ-treated group compared with control group (p < 0.001). Treatment with PSO decreased CK activity in two groups at two times significantly (p < 0.001).
Table 3. Serum level of CK and LDH activity.
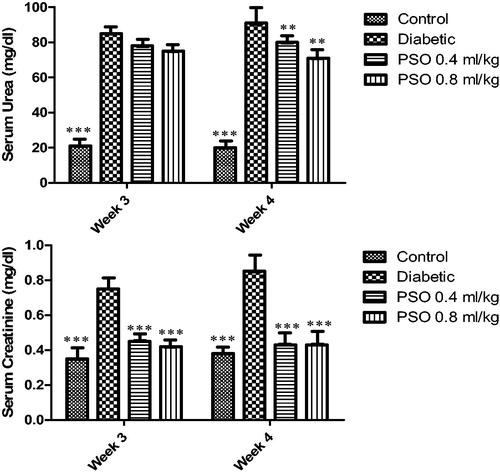
Serum urea and creatinine
STZ caused a marked reduction in renal function, as characterized by significant increases in serum urea and creatinine levels when compared with the control group (p < 0.001). Treatment with PSO significantly reduced serum creatinine (p < 0.01, after 3 and 4 weeks); but reduction in serum urea was not significant (p> 0.05) ().
Urine volume, glucose and protein
The urine volume, glucose and protein levels are shown in . STZ caused a marked reduction in renal function, as characterized by significant increases in urine volume, glucose and protein levels when compared to control group (p < 0.001). PSO treatment resulted in a significant decrease in urinary protein (p < 0.05, after 3 and 4 weeks). Non-significant decrease in urinary glucose compared with STZ-treated group was seen in group III (p> 0.05, at two times of study), but significant decrease was seen in group IV (p < 0.05, at two times of study). Significant decrease in urine volume was seen after treatment with PSO (p < 0.05, at two times of study).
Table 4. Urine volume, glucose and protein.
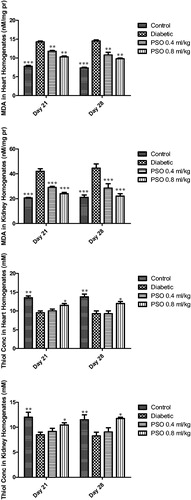
Total thiol content and MDA in tissue homogenates
Total thiol content in tissue homogenates was decreased significantly in STZ-treated group compared to control group (p < 0.01, after 3 and 4 weeks). STZ caused significant elevation in tissue MDA compared to control group (p < 0.01, p < 0.001 in heart and kidney respectively after 3 and 4 weeks). PSO restored total thiol content in both tissues and also decreased MDA levels in both tissue homogenates significantly (p < 0.01 and p < 0.001). Data are shown in ).
Histopathological observations
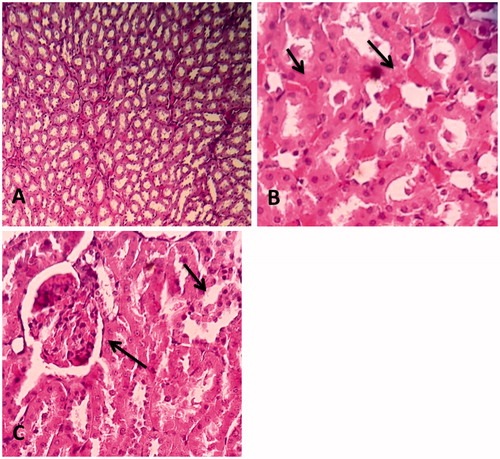
Our findings showed some histopathological changes in the kidney and the heart after 4 weeks of study, but there were no changes after 3 weeks. The renal histopathological damages are shown in . Rats in control group showed normal kidney architecture and histology (A). STZ-treated group showed irregular and widened glomerular capillaries. Severe inflammatory cell infiltration was seen in this group (B). PSO treatment reversed these changes to the normal appearance (C).
Figure 3. Comparison of structural changes in the kidney due to injection of STZ and treatment by PSO between groups. (A) Represents a normal rat kidney. All anatomical structures look normal (×100), (B) group 2 treated with STZ 65 mg/kg, severe inflammatory cells infiltration and hyaline casts (×400), and (C) minimal inflammatory cells infiltration and moderate glomerular congestion have been seen (×400).
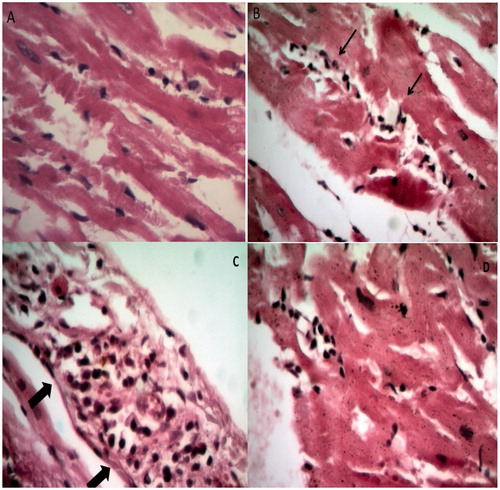
The heart histopathological changes are shown in . Rats in control group showed normal heart architecture and histology (A). Many unspecific changes including hemorrhage, focal inflammation, moderate inflammation of aorta and specific changes including disperse and centralized cardiomyocytes degeneration, inflammatory cells infiltration and pericardial infiltration were significantly observed in STZ-treated group (B and C). Results of PSO-treated groups have shown lesser histopathological findings compared to STZ-treated group (D).
Figure 4. Comparison of structural changes in the heart due to injection of STZ and treatment by PSO between groups. (A) Represents a normal rat cardiomyocyte. All anatomical structures look normal (×400), (B) group 2 treated with STZ 65 mg/kg, disperse and centralized cardiomyocytes degeneration and inflammatory cells infiltration (×400), (C) pericardial infiltration in STZ-treated group (×400), and (D) group 4 treated with PSO 0.8 mL/kg, followed by STZ injection, lesser histopathological findings than STZ-treated group (×400).
Discussion
Oxidative stress due to hyperglycemia is a major cause of the progression of DM and its complications including dyslipidemia, diabetic nephropathy and coronary heart disease (CHD). High amount of superoxide radicals in consequence of chronic hyperglycemia reacts with the nitric oxide and tends to reduce in endothelium-dependent relaxation and cell synthesis in the wall of blood vessels, also, increased levels of inflammatory mediators and glucose oxidation and decreased antioxidant defense due to increased oxidative stress resulting in micro- and macro-pathological changes.Citation26,Citation27
Natural products as an alternative source of medicinal compounds are interested in the researchers’ view point due to their safety, low adverse effects, easy to earn and popularity.Citation8 PSO contains different chemical content including polyphenolic compounds, phytosterols, hydroxybenzoic acid, derivatives of glucopyranoside and conjugated linolenic acids particularly punicic acid and catalpic acid with antioxidant and anti-inflammatory effects.Citation25 Antioxidant and anti-inflammatory properties of PSO have been shown in many studies.Citation28,Citation29 Thus, it is expected that the use of PSO in diabetic condition and excessive oxidative stress status can ameliorate diabetic complications.
In the present study, STZ could induce hyperglycemia as shown in (fasting blood glucose over than 250 mg/dL). The kidney histological changes along with increase in serum urea and creatinine levels, urine volume, excretion of glucose and protein in the urine are indicators of diabetic nephropathy (as shown in and , respectively). Also, heart histological findings with mild elevated serum CK and LDH are indicators of diabetic cardiomyopathy. Several investigations have shown that diabetic nephropathy is associated with lipid peroxidation in renal tissue.Citation30,Citation31 Lipid peroxidation is allied to a free radical-mediated chain reaction that damages cell membranes, and inhibition of this process by PSO is mainly attributed to the ability of scavenging of free radicals. Diabetic nephropathy and cardiomyopathy are the main complications of DM.Citation32 Therefore, significant increase in MDA and significant reduction in total thiol contents in the heart and kidney homogenates are due to the elevated oxidative stress condition and reduced antioxidant enzyme activity which are two major causes of diabetic complication.Citation33,Citation34 In the present study, PSO treatment resulted in a significant reduction in serum urea but not in creatinine levels after 3 and 4 weeks study. The observed lack of protection of PSO against diabetes-induced renal glutathione depletion seems to confirm the failure to thwart the increase in serum creatinine and did not increase creatinine clearance. PSO caused significant reduction in urine protein excretion and urine volume in 3 and 4 weeks study, this is in line with the literature,Citation14 but reduction in urinary glucose was only significant after 4 weeks of the study. A possible reason is shortness of study because DM is a chronic metabolic disease and it might be fruitful that if the study period was longer than four weeks the results may show a significant difference. Also CRP content as a marker of inflammation was positive in STZ-treated group after 4 weeks compared to normal and PSO-treated groups which revealed that PSO could induce anti-inflammatory effect which is well agreed with other studies.Citation35–37 As observed reduction in urine glucose after three weeks was not significant as compared with STZ-treated group, but in contrast, this marker was significant after four weeks of the study as compared with STZ-treated group. Because of progressive weight loss and possible mortality of rats and considering time constraints of the study we urged to finish the test in four weeks. Reduction in serum urea and creatinine in alloxan-induced diabetic rats was seen in Ahmadvand study. In this study, coenzyme Q10 as a potent antioxidant decreased serum urea and creatinineCitation38 and this consequence showed that antioxidant agents such as PSO can be effective to ameliorate this condition. Other studies also have confirmed it.Citation14,Citation28,Citation39
During the experimental period, diabetic animals showed body weight reduction compared to the control group (). This weight loss was inhibited by PSO treatment with a dose of 0.8 mL/kg. Diabetes induced weight loss, already reported by other authors which is proportionated with the percentage of pancreatic islet destruction and serum glucose level.Citation40,Citation41 In our study, prevention of animals weight loss by PSO showed that this agent may be effective in diabetes development.
In this study, PSO reduced serum glucose levels significantly, in a time and dose-dependent manner. Antidiabetic effect of PSO, PA and linolenic acid isomer was shown in some other studies.Citation42–48
Data demonstrated that treatment with PSO could induce protective effect against histopathological changes in the kidney and the heart tissues. These findings revealed that PSO treatment resulted in a better glomerular configuration and capillary shape. Hyaline casts were not seen in the kidney of PSO-treated groups. The results obtained from the kidney histology and also the findings from the heart histology confirm the protective effect of PSO against oxidative stress.
CK and LDH were considered as markers of tissue breakdown, myocardial infarction and cancerous states.Citation49 Elevation of serum CK and LDH in diabetic rats has been shown in Mathew study and treatment of DM has reduced serum levels of these indicators.Citation50 In this study, we speculated the LDH and CK as non-specific markers of cardiomyopathy. PSO reduced serum CK and LDH levels significantly, in a time and dose-dependent manner. We did not measure the LDH-1, as a specific heart subtype of LDH and it was a limitation of our study but the study performed by Khalil et al. showed that the aqueous extract of pomegranate, as a hepatoprotective agent with antioxidant effect reduced the level of serum CK and LDH.Citation51
In diabetic patients, dyslipidemia are accompanied with the risk of CHD, just as they are in the general population.Citation52 Worsen of lipid profile have been shown in DM.Citation53,Citation54 In our study PSO elevated serum HDL level significantly after 4 weeks and decreased significantly serum TG levels in a dose and time dependent manner. Effect of PSO on reduction of LDL was not significant. These results were in agreement with the Mirmiran et al. study.Citation55 In Arao study, punicic acid, a major component of PSO, showed significant reduction in hepatic triacylglycerol but had no effect on HDL and cholesterol levels.Citation16
Conclusion
The results of this study showed that PSO attenuates complications of DM in rats. Reduction in oxidative stress markers, improvement in histopathological findings and amelioration in serum biochemical parameters considered as indicators of protective effects of PSO, but exact mechanism(s) of this protection needs further investigations.
Disclosure statement
The authors declare that there is no conflict of interest.
Funding
This study was financially supported by the Vice Research Chancellery, Mashhad University of Medical Sciences, Mashhad, Iran.
References
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37:S81–S90.
- Elyasi F, Kashi Z, Tasfieh B, Bahar A, Khademloo M. Sexual dysfunction in women with type 2 diabetes mellitus. Iran J Med Sci. 2015;40:206–213.
- Bukhari SA, Javed S, Ali M, Shahzadi A, Rehman M. Serum haematological and biochemical indices of oxidative stress and their relationship with DNA damage and homocysteine in Pakistani type II diabetic patients. Pak J Pharm Sci. 2015;28:881–889.
- Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93:137–188.
- Evans M, Anderson RA, Graham J, et al. Ciprofibrate therapy improves endothelial function and reduces postprandial lipemia and oxidative stress in type 2 diabetes mellitus. Circulation. 2000;101:1773–1779.
- Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: A new perspective on an old paradigm. Diabetes. 1999;48:1–9.
- O’Donnel VB, Chumley PH, Hogg N, Bloodsworth A, Darley-Usmar VM, Freeman BA. Nitric oxide inhibition of lipid peroxidation: Kinetics of reaction with lipid peroxyl radicals and comparison with alpha-tocopherol. Biochemistry. 1997;36:15216–15223.
- Boroushaki MT, Mollazadeh H, Afshari AR. Pomegranate seed oil: A comprehensive review on its therapeutic effects. Int J Pharm Sci Res. 2016;7:430–442.
- Adams LS, Seeram NP, Aggarwal BB, Takada Y, Sand D. Heber pomegranate juice, total pomegranate ellagitannins, and punicalagin suppress inflammatory cell signaling in colon cancer cells. J Agric Food Chem. 2006;54:980–985.
- Goertz A, Ahmad KA. Biological activity of phytochemical compounds in pomegranate – a review. EC Nutr. 2015;1:115–127.
- Vroegrijk IO, Van Diepen JA, Van den Berg S, et al. Pomegranate seed oil, a rich source of punicic acid, prevents diet-induced obesity and insulin resistance in mice. Food Chem Toxicol. 2011;49:1426–1430.
- Boussetta T, Raad H, Lettéron P, et al. Punicic acid a conjugated linolenic acid inhibits TNFalpha-induced neutrophil hyperactivation and protects from experimental colon inflammation in rats. PLoS One. 2009;4:e6458.
- Boroushaki MT, Asadpour M, Sadrghnia HR, Dolati K. Effect of pomegranate seed oil against gentamicin-induced nephrotoxicity in rat. J Food Sci Technol. 2012;51:3510–3514.
- Boroushaki MT, Mollazadeh H, Rajabian A, et al. Protective effect of pomegranate seed oil against mercuric chloride-induced nephrotoxicity in rat. Ren Fail. 2014;36:1581–1586.
- Spilmont M, Léotoing L, Davicco M-J, et al. Pomegranate seed oil prevents bone loss in a mice model of osteoporosis, through osteoblastic stimulation, osteoclastic inhibition and decreased inflammatory status. J Nutr Biochem. 2013;24:1840–1848.
- Arao K, Wang Y-M, Inoue N, et al. Dietary effect of pomegranate seed oil rich in 9cis, 11trans, 13cis conjugated linolenic acid on lipid metabolism in obese, hyperlipidemic OLETF rats. Lipids Health Dis. 2004;3:1–7.
- Al-Sabahi BN, Fatope MO, Essa MM, et al. Pomegranate seed oil: Effect on 3-nitropropionic acid-induced neurotoxicity in PC12 cells and elucidation of unsaturated fatty acids composition. Nutr Neurosci. 2014. [Epub ahead of print]. doi: 10.1179/1476830514Y.0000000155.
- Shaban NZ, El-Kersh MA, El-Rashidy FH, Habashy NH. Protective role of Punica granatum (pomegranate) peel and seed oil extracts on diethylnitrosamine and phenobarbital-induced hepatic injury in male rats. Food Chem. 2013;141:1587–1596.
- Ashafaq M, Varshney L, Khan MH, et al. Neuromodulatory effects of hesperidin in mitigating oxidative stress in streptozotocin induced diabetes. Biomed Res Int. 2014;2014:249031.
- Mollazadeh H, Hosseinzadeh H. The protective effect of Nigella sativa against liver injury: A review. Iran J Basic Med Sci. 2014;17:958–966.
- Masson P, Ohlsson P, Bjorkhem I. Combined enzymic-Jaffé method for determination of creatinine in serum. Clin Chem. 1981;27:18–21.
- Lott JA, Turner K. Evaluation of Trinder's glucose oxidase method for measuring glucose in serum and urine. Clin Chem. 1975;21:1754–1760.
- Mc Elderry LA, Tarbit IF, Cassells-Smith AJ. Six methods for urinary protein compared. J Clin Chem. 1982;28:356–360.
- Hosseinzadeh H, Sadeghnia HR, Ziaee T, Danaee A. Protective effect of aqueous saffron extract (Crocus sativus L.) and crocin, its active constituent, on renal ischemia-reperfusion induced oxidative damage in rats. J Pharm Pharm Sci. 2005;8:387–393.
- Bouroshaki MT, Sadeghnia HR, Banihasan M, Yavari S. Protective effect of pomegranate seed oil on hexachlorobutadiene-induced nephrotoxicity in rat kidneys. Ren Fail. 2010;32:612–617.
- Maritim AC, Sanders RA, Watkins JB 3rd. Diabetes, oxidative stress, and antioxidants: A review. J Biochem Mol Toxicol. 2003;17:24–38.
- Vikram DS, Rivera BK, Kuppusamy P. In vivo imaging of free radicals and oxygen. Methods Mol Biol. 2010;610:3–27.
- Boroushaki MT, Arshadi D, Jalili-Rasti H, Asadpour E, Hosseini A. Protective effect of pomegranate seed oil against acute toxicity of diazinon in rat kidney. Iran J Pharm Res. 2013;12:821–827.
- Mizrahi M, Friedman-Levi Y, Larush L, et al. Pomegranate seed oil nanoemulsions for the prevention and treatment of neurodegenerative diseases: The case of genetic CJD. Nanomedicine. 2014;10:1353–1363.
- Calabrese V, Cornelius C, Leso V, et al. Oxidative stress, glutathione status, sirtuin and cellular stress response in type 2 diabetes. Biochim Biophys Acta. 2012;1822:729–736.
- Das J, Sil PC. Taurine ameliorates alloxan-induced diabetic renal injury, oxidative stress-related signaling pathways and apoptosis in rats. Amino Acids. 2012;43:1509–1523.
- Abdel-Raheem MH, Salim SU, Mosad E, et al. Antiapoptotic and antioxidant effects of carvedilol and vitamin E protect against diabetic nephropathy and cardiomyopathy in diabetic Wistar Albino rats. Horm Metab Res. 2015;47:97–106.
- Elmarakby Ahmed A, Jennifer C. Sullivan Relationship between oxidative stress and inflammatory cytokines in diabetic nephropathy. Cardiovasc Ther. 2012;30:49–59.
- Varga ZV, Giricz Z, Liaudet L, Haskó G, Ferdinandy P, Pacher P. Interplay of oxidative, nitrosative/nitrative stress, inflammation, cell death and autophagy in diabetic cardiomyopathy. Biochim Biophys Acta. 2015;1852:232–242.
- Abidov M, Ramazanov Z, Seifulla R, Grachev S. The effects of Xanthigen™ in the weight management of obese premenopausal women with non‐alcoholic fatty liver disease and normal liver fat. Diabetes Obes Metabol. 2010;12:72–81.
- Jiang Q, Elson-Schwab I, Courtemanche C, Ames BN. gamma-tocopherol and its major metabolite, in contrast to alpha-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc Natl Acad Sci USA. 2000;97:11494–11499.
- Costantini S, Rusolo F, De Vito V, et al. Potential anti-inflammatory effects of the hydrophilic fraction of pomegranate (Punica granatum L.) seed oil on breast cancer cell lines. Molecules. 2014;19:8644–8660.
- Ahmadvand H. Effects of coenzyme Q10 on hemoglobin A1C, serum urea and creatinine in alloxan-induced type 1 diabetic rats. Iran J Pharmacol Ther. 2013;11:64–67.
- Boroushaki MT, Rajabianb A, Farzadnia M, et al. Protective effect of pomegranate seed oil against cisplatin-induced nephrotoxicity in rat. Ren Fail. 2015;37:1338–1343.
- Dekel Y, Glucksam Y, Elron-Gross I, Margalit R. Insights into modeling streptozotocin-induced diabetes in ICR mice. Lab Anim. 2009;38:55–60.
- Cortright RN, Collins HL, Chandler MP, Lemon PW, DiCarlo SE. Diabetes reduces growth and body composition more in male than in female rats. Physiol Behav. 1996;60:1233–1238.
- Anusree S, Nisha V, Priyanka A, Raghu K. Insulin resistance by TNF-α is associated with mitochondrial dysfunction in 3T3-L1 adipocytes and is ameliorated by punicic acid, a PPARγ agonist. Mol Cell Endocrinol. 2015;413:120–128.
- Zolezzi JM, Silva-Alvarez C, Ordenes D, et al. Peroxisome proliferator-activated receptor (PPAR) γ and PPARα agonists modulate mitochondrial fusion-fission dynamics: Relevance to reactive oxygen species (ROS)-related neurodegenerative disorders? PLoS One. 2013;8:e64019.
- Asghari G, Sheikholeslami S, Mirmiran P, et al. Effect of pomegranate seed oil on serum TNF-α level in dyslipidemic patients. Int J Food Sci Nutr. 2012;63:368–371.
- McFarlin BK, Strohacker KA, Kueht ML. Pomegranate seed oil consumption during a period of high-fat feeding reduces weight gain and reduces type 2 diabetes risk in CD-1 mice. Br J Nutr. 2009;102:54–59.
- Nekooeian AA, Eftekhari MH, Adibi S, Rajaeifard A. Effects of pomegranate seed oil on insulin release in rats with type 2 diabetes. Iran J Med Sci. 2014;39:130–135.
- Miranda J, Aguirre L, Fernández-Quintela A, et al. Effects of pomegranate seed oil on glucose and lipid metabolism-related organs in rats fed an obesogenic diet. J Agric Food Chem. 2013;61:5089–5096.
- Baliga MS, Shivashankara AR, Shetty CB, et al. Antidiabetic effects of Punica granatum L. (Pomegranate): A review. In: Watson RR, Preed VR, editors. Bioactive Food as Dietary Interventions for Diabetes. USA: Elsevier; 2013. pp. 355–369.
- Tsitsimpikou C, Kioukia-Fougia N, Tsarouhas K, et al. Administration of tomato juice ameliorates lactate dehydrogenase and creatinine kinase responses to anaerobic training. Food Chem Toxicol. 2013;61:9–13.
- Mathews AM, Christina AJM. Antidiabetic potential of Solanum xanthocarpum Schrad. and wendl. in STZ-nicotinamide induced diabetic rats. World J Pharm Pharm Sci. 2014;3:1378–1386.
- Khalil EAM. A hepatoprotective effect of an aqueous extract of pomegranate (Punica granatum L.) rind against acetaminophen treated rats. Egypt J Hosp Med. 2004;16:112–118.
- Teixeira SR, Tappenden KA, Carson LA, et al. Isolated soy protein consumption reduces urinary albumin excretion and improves the serum lipid profile in men with type 2 diabetes mellitus and nephropathy. J Nutr. 2004;134:1874–1880.
- TODAY Study Group. Lipid and inflammatory cardiovascular risk worsens over 3 years in youth with type 2 diabetes: the TODAY clinical trial. Diabetes Care. 2013;36:1758–1764.
- Luk AO, Lau ES, So WY, et al. Prospective study on the incidences of cardiovascular-renal complications in Chinese patients with young-onset type 1 and type 2 diabetes. Diabetes Care. 2014;37:149–157.
- Mirmiran P, Fazeli MR, Asghari G, Shafiee A, Azizi F. Effect of pomegranate seed oil on hyperlipidaemic subjects: A double-blind placebo-controlled clinical trial. Br J Nutr. 2010;104:402–406.