Abstract
Background: The level and activity of indoleamine 2,3-dioxygenase (IDO) and the concentrations of L-tryptophan and its metabolite L-kynurenine were determined in association with various renal diseases. However, there have been no data regarding these parameters in patients on peritoneal dialysis compared to those undergoing hemodialysis or kidney transplantation.
Methods: This study investigated the level and activity of IDO and determined oxidative balance by calculating the total oxidant status (TOS), total antioxidant status (TAS), and oxidative stress index (OSI). We enrolled 60 kidney disease patients, including 20 on peritoneal dialysis (PD group), 19 on hemodialysis (HD group), and 21 with kidney transplantation (KT group), as well as 21 control group.
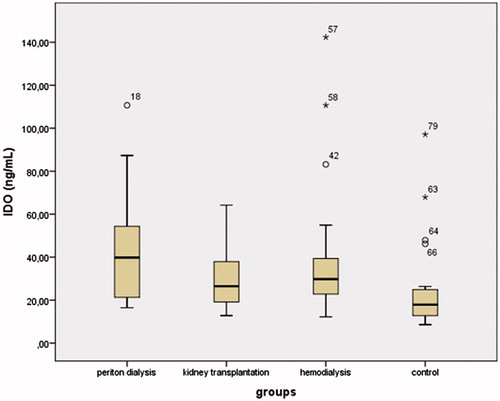
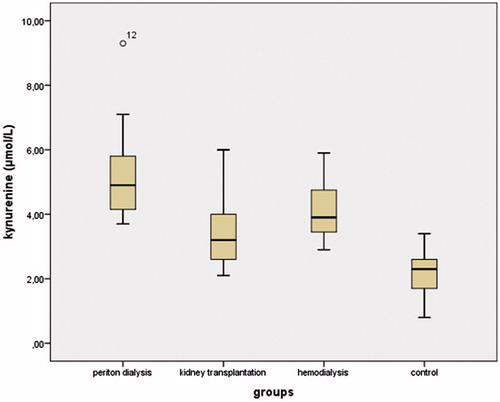
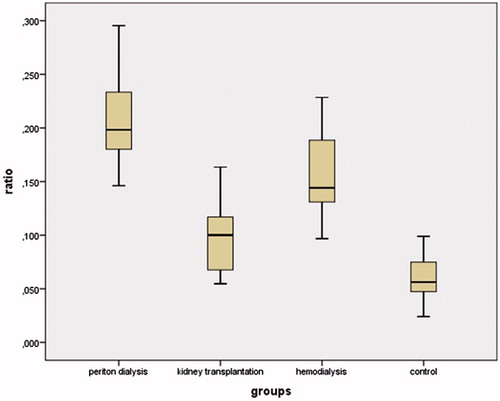
Results: IDO levels were increased in the PD, HD, and KT groups compared to the control group. The concentration of kynurenine was significantly increased in the PD group compared to the other groups (p < 0.01). The kynurenine/tryptophan ratio was increased in the PD group compared to the other groups (all p < 0.01). TAS levels in the PD and HD groups were significantly decreased compared to the control group (both p < 0.05). TAS levels in the PD group were significantly decreased compared to the KT group. TOS levels in the PD group were higher than in the HD and KT groups.
Conclusion: The results showed that IDO levels were increased in peritoneal dialysis and hemodialysis patients and in renal transplant recipients, while oxidative stress was found to be related to IDO activity and was most increased in the patients on peritoneal dialysis.
Introduction
Renal failure is a medical situation in which the kidneys fail to efficiently filter waste yields and cleaning solutes from the blood, eventually leading to death.Citation1 Treatment of renal failure involves the processes of peritoneal dialysis and hemodialysis, and at the end stage of renal failure, renal transplantation may be required.Citation2,Citation3
The immune system involves the deeply complex and crucial regulation of many cytokines.Citation4 Indoleamine 2,3-dioxygenase (IDO) is a substantial regulator of immunity in recurrent bacterial infections and in suppressing the adaptive immune response after transplantation.Citation5 IDO is the first enzyme that converts the essential amino acid tryptophan into kynurenine.Citation6 Tryptophan also has a role in the survival and proliferation of the immune system. Tryptophan and kynurenine levels provide information about IDO activity, which may inhibit activation and proliferation of T cells, natural killer cells, total lymphocytes, and monocytes.Citation7 In addition, IDO may play a critical role in the immunomodulatory enzymes that provide immunosuppression in several diseases, such as cancer, cardiovascular disease, infectious disease, allergic and autoimmune diseases, transplantation, and neuropathology.Citation8–10 Oxidative stress is described as an imbalance between the maintenance of oxidants and antioxidant activity.Citation11 Oxidative stress may be involved in the proinflammatory condition of chronic kidney disease, and elevated urea in dialysis patients may lead to increased oxidant status.Citation12
The current study aimed to study the concentrations of tryptophan and kynurenine in the serum of patients on peritoneal dialysis compared to patients on hemodialysis and those with kidney transplants. The serum expression of IDO was also measured. We examined associations between the activity and level of IDO with oxidative stress status by measuring the total oxidant status (TOS), total antioxidant status (TAS), and oxidative stress index (OSI) in patients on peritoneal dialysis and hemodialysis and in renal transplant recipients. We determined the relationships between IDO activity and expression with age, body mass index (BMI), gender, systolic and diastolic blood pressure, blood urea nitrogen (BUN), and creatinine in patients on peritoneal dialysis compared to those on hemodialysis and kidney transplant recipients.
Materials and methods
Study population
The study included 60 patients with chronic kidney disease, including 20 on peritoneal dialysis (PD group), 19 on hemodialysis (HD group), and 21 with kidney transplants (KT group), as well as 21 healthy control subjects. All participants were age 18 years or older and included individuals on hemodialysis and peritoneal dialysis at Bursa Yuksek Ihtisas Education and Research Hospital, and renal transplant recipients followed at Acibadem Hospital. Patients with cancer, collagen disease, liver disease, thyroid disease, acute infection, pregnancy, lactation, neurologic disease, or drug addiction were excluded from the study. All hemodialysis patients were treated with high-flux hemodialysis on polysulfone membrane dialyzers using bicarbonate-containing solutions for 4 h, three times per week. All patients in the PD group were on continuous ambulatory peritoneal dialysis. The patients mostly underwent 2–3 exchanges with 1.36/1.5% glucose solution and one with 2.27/2.5% glucose solution. Patients also used icodextrin and/or amino acid-containing solutions. The patients remained on renal replacement therapy from eight to 139 months (mean 44.2 ± 32.5 months). The control group was age- and gender-matched to the other groups. The study was approved by the local ethics committee, and informed consent was obtained from all participants. The study protocols were conducted in accordance with the Declaration of Helsinki and were reviewed and accepted by the Institutional Review Board.
Blood sampling
Peripheral venous blood samples (5 mL) were collected by venipuncture into biochemistry tubes and centrifuged for 10 min at 4000 rpm, and the serum was separated and stored at −80 °C until analysis.
Tryptophan and kynurenine analyses
The samples were diluted with 0.1% (v/v) formic acid in 20% (v/v) methanol solution, as previously described by Kivrak et al.Citation13 Tryptophan and kynurenine levels were detected using ultra-performance liquid chromatography (UPLC) (Acquity Ultra Performance LC, Waters Co., Milford, MA) with electrospray ionization (ESI) and tandem mass spectrometry (MS/MS) (Xevo TQ-S MS/MS, Waters Co., Milford, MA), and C18 column (Acquity UPLC BEH C18 100 × 2.1 mm, 1.7 μm particle size). Gradient elution was used to separate the compounds. The mobile phases were composed of solvent A (0.1% formic acid in water) and solvent B (0.1% formic acid in methanol). The flow rate was 0.4 mL·min−1. The operating parameters for the mass spectrometer were a capillary voltage of 2.00 kV and source and desolvation temperatures of 150 °C and 500 °C, respectively. Desolvation and cone gas flow were 1000 and 150 L·h−1, respectively.Citation14,Citation15 The multiple reaction monitoring (MRM) analysis method parameters are shown in . The ESI source was used in positive mode, and cone voltage was 20 V by the MRM mode. All tests were carried out in triplicate. Dwell time was 10 ms for all transitions. Data analysis and quantification were performed using Waters MassLynx 4.1 and TargetLynx software. An 8-point standard curve (including zero) was constructed for each analyte by plotting the peak area of the MRM transitions, giving the most intense signal of each analyte versus its nominal concentration ()). Units of the measurements were given in the legends of the as mg mL−1. Analytical parameters of UPLC-MS/MS method validation are shown in . Data are expressed in μmol/L.
Figure 1. (a) MRM chromatograms and calibration curves of 100 mg mL−1 of tryptophan analyzed using UPLC-ESI-MS/MS. (b) MRM chromatograms and calibration curves of 100 mg mL−1 of kynurenine analyzed using UPLC-ESI-MS/MS.

Table 1. Method parameters for tryptophan and kynurenine using UPLC-ESI-MS/MS.
Table 2. Analytical parameters of UPLC-MS/MS method validation.
IDO analysis
IDO levels were determined using a commercial double-antibody sandwich enzyme-linked immunosorbent assay (ELISA) kit (Sunred Biotechnology Company, Shanghai, China). The plate was coated with specific IDO antibody, and at the end of the reaction, color changes were measured spectrophotometrically at 450 nm. The level of IDO was determined by comparing the optic density to the calibration curve. The level of IDO was expressed as ng/mL.
TAS and TOS measurements
Serum TAS and TOS were determined using an automated measurement method as previously described by Erel.Citation16,Citation17 Absorbance was measured spectrophotometrically using Rel Assay Diagnostics, and results were expressed as μmol Trolox equivalent/L for TAS and mmol H2O2 equiv./L for TOS. Dividing TOS by TAS provided the oxidative stress index (OSI) (TOS, umol H2O2 equiv./L)/(TAS, umol Trolox equiv./L) (arbitrary unit).
Biochemical parameter measurements
BUN, creatinine, total cholesterol, triglycerides, and low-density lipoprotein (LDL), and high-density lipoprotein (HDL) cholesterol were measured with commercially available assay kits (Abbott Diagnostics, Abbott Park, IL) and an Architect C16000 auto-analyzer (Abbott Diagnostics).
Statistical analysis
Data were analyzed using SPSS (statistical package for social sciences) 20.0 (IBM Corp., Armonk, NY) and all data were expressed as mean ± standard deviation (SD). The variables with normal distributions were analyzed using the Kolmogorov–Smirnov test. Student’s t-test and the Mann–Whitney U test were used for comparisons between the groups. The categorical variables between groups were analyzed using the chi-square test. The relationships between continuous variables were evaluated using Pearson’s correlation coefficient. A p value of <0.05 was considered statistically significant.
Results
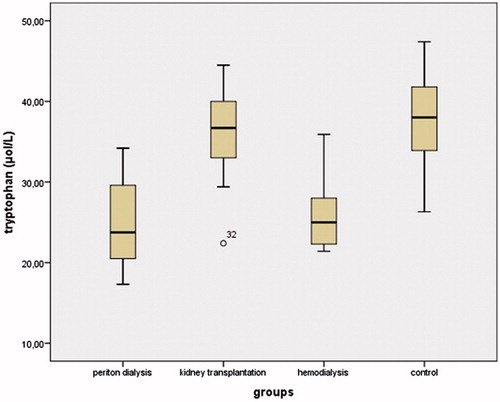
The demographic and biochemical data of the participants are shown in . There were no significant differences in age, gender, BMI, or systolic and diastolic blood pressure between the groups. The serum IDO concentrations in the PD, HD, and KT groups were increased compared to the control group (43.77 ± 26.19, 41.2 ± 34.59, 31.01 ± 16.36, and 25.65 ± 22.03; p = 0.003, p = 0.016, and p = 0.046, respectively) (). The highest IDO concentrations were found in the PD group. There were decreased tryptophan and elevated kynurenine levels in the PD, HD, and KT groups compared to the control group. Tryptophan concentrations in the PD and HD groups were significantly decreased compared to the KT and control groups (both p < 0.05) (). Kynurenine concentrations in the PD group were significantly increased compared to the HD, KT, and control groups (all p < 0.05) (). The kynurenine/tryptophan ratio was significantly increased in the PD group compared to the HD, KT, and control groups (all p < 0.01) ().
Table 3. Comparison of demographic and characteristics of the patients and healthy controls (mean ± SD).
Based on Pearson’s correlation, there was a relationship between kynurenine and TOS, BUN, and creatinine (r = 0.455, p = 0.001; r = 0.617, p = 0.00; and r = 0.545, p = 0.00, respectively). There was an inverse correlation between tryptophan and both BUN and creatinine (r = −0.656, p = 0.00; r = −0.680, p = 0.00, respectively). The kynurenine/tryptophan ratio was strongly correlated to IDO, OSI, BUN, and creatinine (r = 0.263, r = 0.814, r = 0.779, and r = 0.738, respectively; all p = 0.001). IDO concentrations were correlated with BUN (r = 0.245, p = 0.027) and creatinine (r = 0.392, p = 0.000). Age was correlated to BMI (r = 0.335, p = 0.002) and systolic blood pressure (r = 0.340, p = 0.002). Gender was inversely correlated to BUN (r = −0.372, p = 0.001) and creatinine (r = −0.364, p = 0.001) ().
Table 4. The correlations of BUN and creatinine with gender, IDO, KYN, TRP, KYN/TRP ratio (n = 81).
BUN was significantly elevated in the PD group compared to all other groups (p = 0.000), and creatinine was significantly elevated in the PD group compared to the KT and control groups (p = 0.000).
The serum concentration of TAS was significantly decreased in the PD and HD groups compared to the control group (both p < 0.05). TAS levels in the PD group were significantly decreased compared to the KT group. The concentration of TOS in the PD and HD groups was significantly increased compared to the control group (both p < 0.05). TOS levels in the PD group were significantly elevated compared to the HD and KT groups. The kynurenine/tryptophan ratio and kynurenine and urea levels were positively correlated to TOS levels.
Total, LDL, and HDL cholesterol levels were significantly increased in the PD group compared to the KT group (p = 0.005, p = 0.004, and p = 0.020, respectively). HDL cholesterol was significantly higher in the PD group than in the HD group (p = 0.040). Total, LDL, and HDL cholesterol were significantly decreased in the KT group compared to the control group (p = 0.002, p = 0.001, and p = 0.001, respectively). Total and HDL cholesterol were significantly decreased in the HD group compared to the control group (p = 0.038 and p = 0.024, respectively).
Discussion
The present study showed higher serum IDO levels in the PD, HD, and KT groups compared to the healthy controls. This is the first report on patients undergoing peritoneal dialysis compared to hemodialysis and kidney transplant recipients. This is also the first report of increased kynurenine/tryptophan ratios in the PD group compared to the other groups. Decreased tryptophan and increased kynurenine have been described in patients with renal disease, and our data agree with these reports.Citation8,Citation18 We similarly found decreased tryptophan and increased kynurenine levels in the HD and KT groups compared to the control group. However, in this study the tryptophan and kynurenine levels were determined in patients currently on peritoneal dialysis. The kynurenine/tryptophan ratio was positively correlated with OSI, BUN, and creatinine levels. We used a highly sensitive technique, UPLC-MS/MS, to measure tryptophan and its metabolite, kynurenine, in the participants’ serum.Citation19 As previously described, the kynurenine/tryptophan ratio shows the activity of IDO.Citation20 Chronic renal disease is characterized by imbalances in immunity and disruption of the immune response, and IDO is involved in down-regulation in the immune process, as a crucial immunosuppressive molecule.Citation21,Citation22 In inflammation, IDO is elevated in response, and it is also a potential marker of immune activation in cardiovascular disease.Citation23 The measured increased serum IDO concentration could be the result of the increased expression of this enzyme in the monocytes. The value of such a measurement has been confirmed in a study in HD patients in which plasma IDO level was found increased, positively associated with markers of inflammation (indicating that inflammation upregulates IDO), and negatively associated with response to a vaccine with a T-cell dependent antigen.Citation24 Tryptophan is an essential amino acid that is metabolized into kynurenine by IDO. Kynurenine is known as the main metabolic route for the catabolism of tryptophan. Kynurenine is involved in several fundamental biological processes, such as cell apoptosis and interruption of the cell cycle in the G1 phase, and it plays a key role in the regulation of inflammation in some diseases and in oxidative stress.Citation25 It was reported that interleukins and superoxide dismutase can inhibit the activity of IDO. In addition, the synthesis of IDO is induced by inflammatory cytokines. Other inflammatory molecules, such as interferon α and β, may stimulate the activity of IDO.Citation26,Citation27 Moreover, various studies have indicated that IDO has effects on the immunoregulatory mechanism in chronic renal disease.Citation18,Citation20 In context with the previous comment, it is interesting the fact that at the noted serum concentrations neither the decrease in L-tryptophan via general nonderepressible 2 (GCN2) kinase activation, nor the increase in kynurenine via aryl-hydrocarbon receptor (AhR) activation could be responsible for the immunomodulatory effects of IDO since in vitro experiments have shown that much larger alterations are required. However, a recent study in HD patients confirmed increased IDO levels in monocytes, which makes possible its immunomodulatory role in the microenvironment of inflammation, where such alterations are possible.Citation28 In addition, one mechanism by which IDO exerts its immunosuppressive effect is induction of T-cell apoptosis. A study in HD patients detected an inverse relation between plasma IDO levels and T-cell count.Citation29 A proinflammatory process has been identified in patients with chronic uremia, which may induce uremic complications characteristic of disease, such as oxidative stress and vascular and neurologic impairment.Citation27,Citation30 These complications lead to decreased quality of life in dialysis patients. In our study, BUN and creatinine were increased in the PD group compared to the other groups, and IDO was correlated to BUN and creatinine levels. There was a statically significant difference between the PD group and the KT group with regard to BUN and creatinine. In addition, serum BUN levels were significantly increased in the PD group compared to the HD group. After successful transplantation, many metabolic abnormalities associated with uremia may resolve. In the present study, the kidney transplant recipients had no significant differences with the control group with regard to BUN.
Elevated oxidative stress may cause chronic inflammation in patients on hemodialysis and peritoneal dialysis.Citation31 Therefore, there may be a focus on inhibiting or activating IDO, and on oxidative status. In the current study, we determined the activity of IDO and oxidative stress in all of the subjects. It has been shown that IDO activity is increased under oxidative stress conditions. Oxidative stress and infections were the first activated mechanisms of IDO activity.Citation32 Thus, kynurenine may be enhanced during oxidative stress, and the ratio of tryptophan to kynurenine may reflect the condition of oxidative stress in patients with chronic kidney disease who are on hemodialysis and peritoneal dialysis. Oxidative stress might contribute to the decline of renal function in these patients, and it may provoke inflammation via activation of immune cells. Likewise, the presence of inflammation promotes oxidative stress via several mechanisms, including generating reactive oxygen and nitrogen. Inflammation and oxidative stress are correlated to poor clinical outcomes in chronic kidney disease patients undergoing hemodialysis and peritoneal dialysis.Citation31 The present study showed that TOS levels were higher in the PD group than in the HD group, and the TOS levels were increased in both of the dialysis groups compared to the control group. This is similar to several previous reports. Samouilidou et al. reported that TAS and TOS levels were more significantly increased in patients on hemodialysis than in healthy individuals, and hsCRP, an inflammatory marker, was positively correlated to TAS levels. It was suggested that hemodialysis patients have great oxidative stress and, as a defense mechanism, increased antioxidant capacity.Citation33 A connection between oxidative stress and inflammation in hemodialysis patients may exist.
Clinically, waste substances in the blood are used for the determination of renal function with regard to BUN and urea.Citation34 However, new markers have been investigated, and the kynurenine/tryptophan ratio has been identified as a sensitive and reliable marker for renal function.Citation26 The kynurenine/tryptophan ratio has also been recommended as a strong and crucial marker to observe the activation of IDO.Citation20 In our study, the kynurenine/tryptophan ratio was the most increased in the PD group, seeming to indicate that peritoneal dialysis is not a good treatment for chronic renal disease. It was also of note that kynurenine was found to positively correlate with TOS levels, with an increased kynurenine/tryptophan ratio being related to oxidative stress. The presence of oxidative stress in chronic renal disease might induce the proinflammatory condition as a result of the increased kynurenine/tryptophan ratio. These findings are also consistent with the elevated kynurenine and kynurenine/tryptophan ratios found to be common in hemodialysis patients in previous studies, such as that of Kato et al. Tryptophan was decreased in the HD group compared to the control group in the present study.Citation35 The accumulation of tryptophan metabolites contributes to several outcomes, such as infectious disease, anemia, and neurological disease.Citation20
The treatment for end-stage chronic renal disease is kidney transplantation, which provides improved mortality and quality of life compared to peritoneal dialysis and hemodialysis.Citation36 The oxidant–antioxidant balance in patients on dialysis and with renal transplants has been determined in several studies, with some showing that oxidative stress was increased and counterbalanced by elevated antioxidant mechanisms in kidney transplant recipients.Citation37 In one study, IDO activity was related to graft survival in transplant patients.Citation38 In another study, cholesterol levels were increased in the PD group compared to the transplant group.Citation39 Zinellu et al. reported that cholesterol-lowering treatments ameliorated oxidation and inflammation. Elevated cholesterol appears to excite oxidative stress and proinflammatory markers in patients on peritoneal dialysis.Citation40 Brandacher et al. reported that an elevated kynurenine/tryptophan ratio was associated with acute kidney rejection.Citation41 Another report indicated that the induced activity of IDO was not able to prevent the immune response resulting in damage to graft tissue.Citation42 Our data are consistent with the findings of Minz et al. on oxidative status in stable renal transplant recipients, counterbalanced by increased antioxidant pathways.Citation43
In conclusion, patients on peritoneal dialysis, hemodialysis, and kidney transplantation have increased IDO, with the greatest increases in patients on peritoneal dialysis, who were more prone to oxidative stress compared to the other groups. This study highlights the greater importance of oxidative stress and IDO activity in patients on peritoneal dialysis than in those on hemodialysis or with kidney transplants.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Lima SM, Otoni A, Sabino AP, et al. Inflammation, neoangiogenesis and fibrosis inperitoneal dialysis. Clin Chim Acta. 2013;421:46–50.
- Tyan YC, Su SB, Ting SS, Wang HY, Liao PC. A comparative proteomics analysis of peritoneal dialysate before and after the occurrence of peritonitis episode by mass spectrometry. Clin Chim Acta. 2013;420:34–44.
- Ghahramani N, Shadrou S, Hollenbeak C. A systematic review of continuous renal replacement therapy and intermittent haemodialysis in management of patients with acute renal failure. Nephrology (Carlton). 2008;13:570–578.
- Imig JD, Ryan MJ. Immune and inflammatory role in renal disease. Compr Physiol. 2013;3:957–976.
- Mbongue JC, Nicholas DA, Torrez TW, Kim NS, Firek AF, Langridge WH. The role of indoleamine 2,3-dioxygenase in immune suppression and autoimmunity. Vaccines (Basel). 2015;3:703–729.
- Ball HJ, Yuasa HJ, Austin CJ, Weiser S, Hunt NH. Indoleamine 2,3-dioxygenase-2: A new enzyme in the kynurenine pathway. Int J Biochem Cell Biol. 2009;41:467–471.
- Schröcksnadel K, Wirleitner B, Winkler C, Fuchs D. Monitoring tryptophan metabolism in chronic immune activation. Clin Chim Acta. 2006;364:82–90.
- Tanaka A, Kato A, Suzuki Y, et al. Association of increased indoleamine 2,3-dioxygenase with impaired natural killer cell activity in hemodialysis patients. Ther Apher Dial. 2014;18:19–23.
- Suzuki Y, Miwa S, Akamatsu T, et al. Indoleamine 2,3-dioxygenase in the pathogenesis of tuberculous pleurisy. Int J Tuberc Lung Dis. 2013;17:1501–1506.
- Suzuki Y, Suda T, Asada K, et al. Serum indoleamine 2,3-dioxygenase activity predicts prognosis of pulmonary tuberculosis. Clin Vaccine Immunol. 2012;19:436–442.
- Rahal A, Kumar A, Singh V, et al. Oxidative stress, prooxidants, and antioxidants: the interplay. Biomed Res Int. 2014;2014:761264.
- Cachofeiro V, Goicochea M, de Vinuesa SG, Oubiña P, Lahera V, Luño J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int Suppl. 2008;111:S4–S9.
- Kıvrak İ, Kıvrak Ş, Harmandar M. Free amino acid profiling in the giant puffball mushroom (Calvatia gigantea) using UPLC-MS/MS. Food Chem. 2014;158:88–92.
- Kıvrak İ. Analytical methods applied to assess chemical composition, nutritional value and in vitro bioactivities of Terfezia olbiensis and Terfezia claveryi from Turkey. Food Anal Meth. 2015;8:1279–1293.
- Kıvrak İ. Free amino acid profiles of 17 Turkish unifloral honeys. J Liquid Chromatogr Rel Technol. 2015;38:855–862.
- Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–1111.
- Erel O. A novel automated method to measure total anti-oxidant response against potent free radical reactions. Clin Biochem. 2004;37:112–119.
- Eleftheriadis T, Antoniadi G, Liakopoulos V, Stefanidis I, Galaktidou G. Plasma indoleamine 2,3-dioxygenase concentration is increased in hemodialysis patients and may contribute to the pathogenesis of coronary heart disease. Ren Fail. 2012;34:68–72.
- Li S, Teng L, Liu W, et al. Pharmacokinetic study of harmane and its 10 metabolites in rat after intravenous and oral administration by UPLC-ESI-MS/MS. Pharm Biol. 2016. [Epub ahead of print]. doi:10.3109/13880209.2015.1127978.
- Bao YS, Ji Y, Zhao SL, Ma LL, Xie RJ, Na SP. Serum levels and activity of indoleamine 2,3-dioxygenase and tryptophanyl-tRNA synthetase and their association with disease severity in patients with chronic kidney disease. Biomarkers. 2013;18:379–385.
- Kato S, Chmielewski M, Honda H, et al. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol. 2008;3:1526–1533.
- He Y, Zhou S, Liu H, et al. Indoleamine 2,3-dioxgenase transfected mesenchymal stem cells induce kidney allograft tolerance by increasing the production and function of regulatory T cells. Transplantation. 2015;99:1829–1838.
- Polyzos KA, Ketelhuth DF. The role of the kynurenine pathway of tryptophan metabolism in cardiovascular disease. An emerging field. Hamostaseologie. 2015;35:128–136.
- Eleftheriadis T, Liakopoulos V, Antoniadi G, Stefanidis I, Galaktidou G. Indoleamine 2,3-dioxygenase is increased in hemodialysis patients and affects immune response to hepatitis B vaccination. Vaccine. 2011;29:2242–2247.
- Zhao J. Plasma kynurenic acid/tryptophan ratio: a sensitive and reliable biomarker for the assessment of renal function. Ren Fail. 2013;35:648–653.
- Comai S, Bertazzo A, Ragazzi E, Caparrotta L, Costa CV, Allegri G. Influence of age on Cu/Zn-superoxide dismutase and indole 2,3-dioxygenase activities in rat tissues. Ital J Biochem. 2005;54:232–239.
- Praschberger M, Hermann M, Wanner J, et al. The uremic toxin indoxyl sulfate acts as a pro- or antioxidant on LDL oxidation. Free Radic Biol Med. 2014;75:1–36.
- Eleftheriadis T, Pissas G, Antoniadi G, et al. Increased indoleamine 2,3-dioxygenase in monocytes of patients on hemodialysis. Iran J Kidney Dis. 2016;10:91–93.
- Eleftheriadis T, Yiannaki E, Antoniadi G, et al. Plasma indoleamine 2,3-dioxygenase and arginase type I may contribute to decreased blood T-cell count in hemodialysis patients. Ren Fail. 2012;34:1118–1122.
- Vellanki K, Bansal VK. Neurologic complications of chronic kidney disease. Curr Neurol Neurosci Rep. 2015;15:50.
- Tucker PS, Scanlan AT, Dalbo VJ. Chronic kidney disease influences multiple systems: describing the relationship between oxidative stress, inflammation, kidney damage, and concomitant disease. Oxid Med Cell Longev. 2015;2015:806358.
- Wang Q, Liu D, Song P, Zou MH. Tryptophan-kynurenine pathway is dysregulated in inflammation, and immune activation. Front Biosci (Landmark Ed). 2015;20:1116–1143.
- Samouilidou E, Grapsa E, Karpouza A, Lagouranis A. Reactive oxygen metabolites: a link between oxidative stress and inflammation in patients on hemodialysis. Blood Purif. 2007;25:175–178.
- Filiopoulos V, Hadjiyannakos D, Vlassopoulos D. New insights into uric acid effects on the progression and prognosis of chronic kidney disease. Ren Fail. 2012;34:510–520.
- Kato A, Suzuki Y, Suda T, et al. Relationship between an increased serum kynurenine/tryptophan ratio and atherosclerotic parameters in hemodialysis patients. Hemodial Int. 2010;14:418–424.
- Palafox D, Llorente L, Alberú J, et al. The role of indoleamine 2,3 dioxygenase in the induction of immune tolerance in organ transplantation. Transplant Rev (Orlando). 2010;24:160–165.
- Campise M, Bamonti F, Novembrino C, et al. Oxidative stress in kidney transplant patients. Transplantation. 2003;76:1474–1478.
- Ingelsten M, Gustafsson K, Oltean M, Karlsson-Parra A, Olausson M, Haraldsson B. Is indoleamine 2,3dioxygenase important for graft acceptance in highly sensitized patients after combinedauxiliary liver-kidney transplantation? Transplantation. 2009;88:911–919.
- Mulley WR, Nikolic-Paterson DJ. Indoleamine 2,3-dioxygenase in transplantation. Nephrology (Carlton). 2008;13:204–211.
- Zinellu A, Sotgia S, Mangoni AA, Sanna M, Satta AE, Carru C. Impact of cholesterol lowering treatment on plasma kynurenine and tryptophan concentrations in chronic kidney disease: relationship with oxidative stress improvement. Nutr Metab Cardiovasc Dis. 2015;25:153–159.
- Brandacher G, Cakar F, Winkler C, et al. Non-invasive monitoring of kidney allograft rejection through IDO metabolism evaluation. Kidney Int. 2007;71:60–67.
- Serbecic N, Lahdou I, Scheuerle A, Höftberger R, Aboul-Enein F. Function of the tryptophan metabolite, L-kynurenine, in human corneal endothelial cells. Mol Vis. 2009;15:1312–1324.
- Minz M, Heer M, Arora S, Sharma A, Khullar M. Oxidative status in stable renal transplantation. Transplant Proc. 2006;38:2020–2021.