Abstract
Disturbances in hemostasis are common complications of kidney diseases and correlate well with cardiovascular mortality. Little is known about the effects of fasudil on tissue factor (TF) and plasminogen activator inhibitor-1 (PAI-1) expression in peripheral blood mononuclear cells (PBMCs) in CAPD patients. PBMCs were isolated from 13 individuals with CAPD and 13 healthy subjects. After 4 h of incubation with or without LPS (10 ng/mL), TF and PAI-1 mRNA of PBMCs were detected by RT-PCR. The levels of TF and PAI-1 in culture supernatants of PBMCs were determined by ELISA. Compared with healthy controls, CAPD patients had increased TF, PAI-1 protein and mRNA expression by PBMCs at baseline and after stimulated by LPS (10 ng/mL) [p < 0.001]. The fasudil treatment resulted in a significant effect in decreasing TF and PAI-1 [p < 0.05] synthesis in PBMCs. TF and PAI-1 mRNA expression and activities in PBMCs were increased in CAPD patients. Fasudil reduced LPS-mediated TF and PAI-1 expression and activity in PBMCs. These effects may partially be relevant to the clinical benefits of fasudil in the treatment of CAPD patients.
Introduction
It is apparent that hemostatic abnormalities are closely associated with increased atherosclerosis risk and correlate well with cardiovascular mortality.Citation1 Due to the significant increase in cardiovascular morbidity and mortality in dialyzed patients,Citation2,Citation3 more attention has been paid to the role of thrombogenic factors in the pathogenesis of cardiovascular events in uremia.Citation4
Disturbances in hemostasis are common complications of kidney diseases. Their occurrence and severity correlate with the progressive loss of renal function to end-stage renal disease. Uremic patients seem to have two opposite aspects in hemostatic status, which are bleeding diathesis and thromboembolism.Citation5 Clinical statistics suggest that uremic patients have a high incidence of thrombotic events.Citation6,Citation7 A thrombotic tendency, caused by factors such as platelet hyperaggregability and hypercoagulability, has been described in dialysis patients.Citation8,Citation9 Moreover, the relationship between hypoalbuminemia and hemostatic factors has been suggested that the elevated fibrinogen levels in CAPD patients could be induced by protein loss, which in turn could stimulate hepatic synthesis of fibrinogen.Citation10
Tissue factor (TF), a transmembrane cell surface glycoprotein located on the surfaces of certain cell types, is generally held to be the physiological trigger of coagulation in normal hemostasis and ultimately leads to thrombin formation. It activates both intrinsic and extrinsic coagulation pathways. Excessive expression of TF occurs in prothrombotic conditions such as sepsis, endotoxemia, systemic lupus erythematosus, atherosclerosis, Crohn’s disease, transplant rejection reactions and hemolytic uremic syndrome (HUS).Citation11,Citation12 In addition, TF-induced coagulation plays an important role in the pathophysiology of many diseases, including atherosclerosis, thrombosis, ischemia-reperfusion injury, sepsis, or glomerulonephritis.Citation13 TF-dependent coagulation and the concentrations of TF are significantly higher in CAPD patients compared to the healthy volunteers.Citation14,Citation15
Several longitudinal cohort studies have also provided evidence that impaired fibrinolysis due to increased PAI-l activity is implicated in the pathogenesis of atherosclerotic disease. Recently, an enhanced PAI-l expression was observed in atherosclerotic coronary arteries with acute coronary thrombosis, and it was suggested that an increased PAI-l level may precipitate thrombosis in cases of sudden cardiac death.Citation16 CAPD patients with atherosclerosis had significantly higher PAI-l levels than those without atherosclerosis and the normal controls.Citation9
The small GTPase, RhoA, belongs to the Rho subfamily and has been implicated in many cellular functions, such as cell adhesion, cell motility and migration, growth control, cell contraction, and cytokinesis. One of its main effectors, Rho-kinase (ROCK), has two known isoforms (ROCK1 and ROCK2) and regulates cytoskeletal reorganization by phosphorylating myosin phosphatase, which results in an increase in myosin light chain (MLC) phosphorylation.Citation17,Citation18
The aims of this study were to investigate the effects of fasudil on TF and PAI-1 expression in peripheral blood mononuclear cells in CAPD patients.
Materials and methods
Patients
The study protocol was approved by the Ethics Committee on Human Studies at Hainan General Hospital, Hainan, Haikou, China. Informed consent was obtained from each patient. Thirteen uremic patients undergoing CAPD with standard glucose solutions for more than 6 months were enrolled in this study. They were 10 men and 3 women with ages ranging from 25 to 71 years (49.92 ± 14 years). Duration of CAPD treatment ranged from 6 to 60 months (mean, 22.23 months). Causes of renal failure were chronic glomerulonephritis in 7 patients, hypertensive nephropathy in 1 patient, obstructive nephropathy in 1 patient, and Diabetic nephropathy in 4 patients. No patient had experienced peritonitis in the past 6 months and no liver dysfunction was observed (prothrombin time, alanine, and asparaginase aminotransferases within normal range). Patients with acute illnesses were excluded. All patients performed three 4-h 2-L exchanges of 1.5% glucose solution during the day and one 9-h 2-L exchange of 1.5% or 2.5% glucose solution while sleeping at night. They continued their regular medications, such as antihypertensives, erythropoietin, and phosphate binders, except for lipid lowering agents. Their current medications are listed in . The control group consisted of 13 healthy subjects with sex and age distributions similar to those of the patient group.
Table 1. Characteristics of participants.
Isolation of PBMCs
PBMCs were collected from all participants after an overnight fast (14 h). Fifteen milliliters of peripheral blood were drawn into sterile 25 ml tubes containing sodium heparin and centrifuged at 1500g for 20 min. Cells were harvested and washed three times in RPMI1640 medium (Gibco-BRL, Grand Island, NY) containing 2 mmol/L glutamine (Sigma) and 10% heat-inactivated fetal calf serum (Gibco-BRL). Cells were suspended at 1 × 106/ml in RPMI1640 (Gibco-BRL, Grand Island, NY) in 12-well culture plates and 1h-preincubation with 10μM fasudil (Biotang Inc., Lexington, MA), then stimulated with or without 10 ng/mL LPS for 4 hours. Cell viability was always >95%, as estimated by trypan blue exclusion. The cell suspension was plated at 2 mL per well in 12-well flat-bottomed tissue culture plates (Costar, Cambridge, MA). After 4 h of incubation with or without LPS (10 ng/mL) (Sigma-Aldrich, St. Louis, MO) at 37 °C in 95% humidified air and 5% CO2, cell supernatants were harvested and stored at −70 °C for cytokine analysis.
Activity of TF and PAI-1 in culture supernatants of PBMCS
The TF and PAI-1 assay were quantified by enzyme-linked immunosorbent assay (ELISA) (Shanghai JingMa Biotechnology, China). TF and PAI-1 concentrations were determined by measuring absorbance at 450 nm.
RNA extraction and reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA of equivalence PBMCs was isolated by using TRIzol reagent (Gibco-BRL) according to the manufacturer's instructions. Equal amounts of RNA were analyzed for TF, PAI-1, glyceraldehydes-3-phosphate dehydrogenase (GAPDH) mRNA concentrations by quantitative reverse transcription-polymerase chain reaction (RT-PCR). The sequences of the sense and antisense primers used for amplification were as follows: TF 5′-AAGCAGTGATTCCCTCTCG-3 and 5′-AACACAGCATTGGCAGCAG-3′; PAI-15′-ATTGCTGCCCCTTATGAAAA-3′ and 5′-G CCAAGGTCTTGGAGACAGA-3′,GAPDH (internal control) 5′-GGAGC CAAAAGGGTCA TC-3′ and 5′- CCAGTGAGTTTCCCGTTC-3′.Citation19,Citation20 PCR cycle for TF (254 bp) and GAPDH (346 bp) consisted of denaturing at 94 °C for 50 s, annealing at 58 °C for 50 s, and elongation at 72 °C for 60 s, conducted for 39 cycles. PCR cycle for PAI-1 (596 bp) and GAPDH consisted of denaturing at 94 °C for 50 s, annealing at 55 °C for 50 s, and elongation at 72 °C for 60 s, conducted for 38 cycles. Those PCR products were electrophoresed on 1.5% agarose gel. Densitometric measurements were made, and the relative density (normalized by the amount of internal control) was given.
Statistical analysis
Results were expressed as mean ± SD Differences between groups were evaluated by the Student's unpaired/paired two-tailed t-test. Statistically significant differences between groups were reported when p ≤ 0.05.
Results
Characteristics of the subjects
The clinical and biochemical characteristics of CAPD patients and healthy individuals are shown in . summarizes the medications of 13 CAPD patients.
Table 2. Medications of 15 CAPD patients.
Secretion of TF and PAI-1 into culture supernatants by PBMCs
At baseline, spontaneous secretion of TF and PAI-1 into culture supernatants by PBMCs differed significantly between CAPD patients and healthy controls (p < 0.001). TF and PAI-1 secretion stimulated by LPS were significantly higher in patients with CAPD vs. spontaneous secretion of healthy individuals (p < 0.001) ().
Table 3. Secretion of TF and PAI-1 by PBMCs at baseline and stimulated by LPS.
The changes of TF and PAI-1 concentrations stimulated by LPS after treatment with fasudil are shown in . It showed that Fasudil treatment resulted in a significant effect in decreasing TF and PAI-1 secretion in PBMCs (p < 0.05).
TF and PAI-1 mRNA concentrations in PBMCs
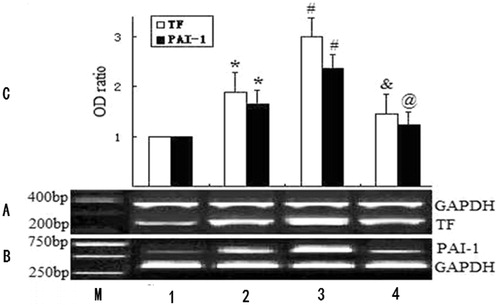
Experiments were performed to compare the concentration of gene expression in PBMCs between control and CAPD. TF mRNA concentrations in PBMCs from LPS-treated group were significantly increased when compared with the control, LPS and fasudil-treated group (p < 0.05, ). PAI-1 mRNA concentrations in PBMCs from LPS-treated group were significantly increased when compared with the control, LPS and fasudil-treated group (p < 0.05, ).
Figure 1. mRNA expression of TF and PAI-1 in PBMCs. Control indicates normal group; (A) RT-PCR analysis of TF mRNA expression (upper bands: GAPDH mRNA,346 bp; lower bands: TF mRNA, 254 bp); (B) RT-PCR analysis of PAI-1 mRNA expression (upper bands: PAI-1 mRNA, 596 bp; lower bands: GAPDH mRNA,346 bp); M: Marker; lane 1: normal group; lane 2: CAPD group; lane 3: LPS-treated group; lane 4: LPS and fasudil-treated group. (C) Determination of relative signal intensity of TF and PAI-1 expression. Bar graph represents quantification of TF/GAPDH or PAI-1/GAPDH mRNA signals of 3 reproductions of independent experiment. For each group, mean ± SD. *p < 0.05 health group vs. CAPD group. #p < 0.05 CAPD group vs. LPS-treated CAPD group. &p < 0.05 LPS-treated CAPD group vs. LPS and fasudil-treated CAPD group. @p < 0.01 LPS-treated CAPD group vs. LPS and fasudil-treated CAPD group.
Discussion
Disturbances in hemostasis are common findings in renal abnormalities patients. Both bleeding diathesis and hypercoagulable state are observed. The principal cause of these abnormalities is the uremic state.Citation15 Our present study provides evidence that these abnormalities might be partly due to the increased TF and PAI-1 synthesis by PBMCs.
Patients on CAPD showed evidence of a higher degree of hypercoagulation.Citation15 Significant protein losses through the peritoneum, including fibrinolytic activators, may be counterbalanced by the possible increase in protein synthesis in CAPD. The intensity of hypercoagulability is thought to be related to the degree of hypoalbuminemia.Citation20 Koyama et al. reported that plasma TF concentrations were increased in uremic patients on chronic dialysis.Citation21 PAI-1 levels in HD and CAPD patients are similar to healthy controls, and this supports many earlier studies.Citation22,Citation23 In contrast, Goedde et al.Citation24 reported high blood levels of PAI-1 during CAPD. Our study showed that TF and PAI-1 mRNA expression and activities in PBMCs were increased in CAPD patients when compared with the healthy subjects. LPS-mediated TF and PAI-1 mRNA expression and activities in PBMCs were further upregulated.
In the present study, we first demonstrated that fasudil inhibited LPS-mediated TF and PAI-1 secretion by PBMCs in CAPD patients. The Rho/ROCK-mediated pathway plays a role in infiltration of Z cells both in vitro and in vivo.Citation25 It is reported that Rho-kinase signaling plays a central role in LPS-mediated leukocyte- endothelial cell interactions. Thus, Rho-kinase inhibition might be useful in the prevention or treatment of pathological inflammation and endotoxin-mediated hypercoagulation.Citation26 Meanwhile, fasudil inhibited PAI-1 mRNA and protein expression in bleomycin-induced pulmonary fibrosis.Citation27
Fasudil reduced LPS-mediated TF and PAI-1 expression and activity in PBMCs. These effects may partially be relevant to the clinical benefits of fasudil in the treatment of CAPD patients.
| Abbreviations | ||
| CAPD | = | continuous ambulatory peritoneal dialysis |
| LPS | = | lipopolysaccharide |
| TF | = | tissue factor |
| PAI-1 | = | plasminogen activator inhibitor-1 |
| PBMC | = | peripheral blood mononuclear cells |
Disclosure statement
The authors declare that they have no competing interests.
Funding
This project was supported by Hainan Social Development Projects [2015SF43], National Science Foundation of China [81560124] and Hainan health and planned parenthood project [13A210277].
References
- Dubin R, Cushman M, Folsom AR, et al. Kidney function and multiple hemostatic markers: Cross sectional associations in the multi-ethnic study of atherosclerosis. BMC Nephrol. 2011;12:3.
- Goluza E, Topalović MG, Hudolin T, Konosić S, Kocman IB, Perić M. Disorders of hemostasis in chronic renal failure and renal transplantation. Acta Med Croatica. 2011;65:337–347.
- Poulikakos D, Banerjee D, Malik M. Risk of sudden cardiac death in chronic kidney disease. J Cardiovasc Electrophysiol. 2014;25:222–231.
- Chitalia VC, Shivanna S, Martorell J, et al. Uremic serum and solutes increase post-vascular interventional thrombotic risk through altered stability of smooth muscle cell tissue factor. Circulation. 2013;127:365–376.
- Tanaka H, Sonoda M, Kashima K, et al. Impact of decreased renal function on coagulation and fibrinolysis in patients with non-valvular atrial fibrillation. Circ J. 2009;73:846–850.
- Ansari I, Sheikh A, Ahmed SS, Jabbar Q, Ali S. Management of anemia and other hematologic derangements in patients with chronic kidney disease. Arab J Nephrol Transplant. 2014;7:13–19.
- London GM, Druke TB. Atherosclerosis and arteriosclerosis in chronic renal failure. Kidney Int. 1997;51:1678–1695.
- Wand S, Schneider S, Meybohm P, Zacharowski K, Weber CF. Assessment of hemostatic changes after initiation of continuous venovenous hemodialysis. Clin Lab. 2015;61:379–387.
- Preloznik Zupan I, Sabovic M, Salobir B, Buturovic Ponikvar J. Characterization of the pro-thrombotic state in CAPD patients. Ren Fail. 2008;30:597–602.
- Bartens W, Nauck M, Schollmeyer P, Wanner C. EIevated lipoprotein (a) and fibrinogen serum levels increase the cardiovascular risk in continuous ambulatory peritoneal dialysis patients. Perit Dial Int. 1996;16:27–33.
- Yamani MH, Starling RC, Young JB, et al. Acute vascular rejection is associated with up-regulation of vitronectin receptor (aVb3), increased expression of tissue factor, and activation of the extracellular matrix metalloproteinase induction system. J Heart Lung Transplant. 2002;21:983–989.
- Stahl AL, Vaziri-Sani F, Heinen S, et al. Factor H dysfunction in patients with atypical hemolytic uremic syndrome contributes to complement deposition on platelets and their activation. Blood. 2008;111:5307–5315.
- Broze GJ Jr. Tissue factor pathway inhibitor. Thromb Haemost. 1995;74:90–93.
- Malyszko J, Malyszko JS, Mysliwiec M. Comparison of hemostatic disturbances between patients on CAPD and patients on hemodialysis. Perit Dial Int. 2001;21:158–165.
- Pawlak K, Mysliwiec M, Pawlak D. Hypercoagulability is independently associated with kynurenine pathway activation in dialysed uraemic patients. Thromb Haemost. 2009;102:49–55.
- Karásek D, Vaverková H, Halenka M, Slavík L, Novotný D. Endothelial haemostatic markers in members of families with familial combined hyperlipidemia. Thromb Res. 2009;123:466–475.
- Ha BH, Morse EM, Turk BE, Boggon TJ. Signaling, regulation, and specificity of the type II p21-activated kinases. J Biol Chem. 2015;290:12975–12983.
- Julian L, Olson MF. Rho-associated coiled-coil containing kinases (ROCK): Structure, regulation, and functions. Small GTPases. 2014;5:e29846.
- Aikawa M, Voglic SJ, Sugiyama S, et al. Dietary lipid lowering reduces tissue factor expression in rabbit atheroma. Circulation. 1999;100:1215–1122.
- Pretorius E, Lipinski B, Bester J, Vermeulen N, Soma P. Albumin stabilizes fibrin fiber ultrastructure in low serum albumin type 2 diabetes. Ultrastruct Pathol. 2013;37:254–257.
- Koyama T, Nishida K, Ohdama S, et al. Determination of plasma tissue factor antigen and its clinical significance. Br J Hematol. 1994;84:343–347.
- Kobayashi M, Yorioka N, Yamakido M. Hypercoagulability and secondary hyper-fibrinolysis may be related to abnormal lipid metabolism inpatients treated with continuous ambulatory peritoneal dialysis. Nephron. 1997;76:56–61.
- Al-Wakeel J, Gader AM, Huraib S, et al. Coagulation inhibitors and fibrinolytic parameters in patients on peritoneal dialysis and haemodialysis. Int Urol Nephrol. 1996;28:255–261.
- Goedde M, Siltter T, Schiffl H, Bechtel U, Schram W, Spangl M. Coagulation- and fibrinolysis-related antigens in plasma and dialysate of CAPD patients. Perit Dial Int. 1997;17:162–170.
- Zanin-Zhorov A, Waksal SD. ROCKing cytokine secretion balance in human T cells. Cytokine. 2015;72:224–225.
- Slotta JE, Braun OO, Menger MD, Thorlacius H. Central role of rho kinase in lipopolysaccharide-induced platelet capture on venous endothelium. J Investig Med. 2008;56:720–725.
- Jiang C, Huang H, Liu J, Wang Y, Lu Z, Xu Z. Fasudil, a rho-kinase inhibitor, attenuates bleomycin-induced pulmonary fibrosis in mice. Int J Mol Sci. 2012;13: 8293–8307.
