Abstract
Introduction: There are some evidences indicating DNA damage by oxidant and mutant agents has an essential role in the chronic renal failure and end stage renal disease (ESRD). To investigate the possible association of GSTs variants with ESRD, we investigated the frequency of GST- T1, M1, and P1 genotypes, and the level of malondialdehyde (MDA) in patients with ESRD.
Materials and methods: The present case-control study consisted of 136 ESRD patients treated with maintenance hemodialysis and 137 gender- and age-matched, unrelated healthy controls from the population of west of Iran. The GST- T1, M1, and P1 genotypes were determined in all individuals using multiplex-PCR and PCR-RFLP. The level of MDA was measured by high-performance liquid chromatography (HPLC).
Results: We found that GSTM1 and GSTT1 null genotypes (GSTT1−/GSTM1−) increased the risk of ESRD by 1.8 times (p < 0.001) and the increased risk of ESRD for GSTM-null (T1+-M1−) genotype was 3.04 times (p = 0.002). ESRD patients carriers the GST (GSTM1-null + GSTT1-null + GST-null) genotypes compared to GST normal genotype increased the risk of ESRD by 3.3 (p < 0.001) times. ESRD patients carriers of GST-null, GSTM1-null, and GSTT1-null genotypes had greater MDA concentration compared with the same genotypes of control subjects. Our results indicated that the GST-null allele (GSTT1-null/GSTM1-null) is a risk factor for ESRD and carriers of this allele have high levels of MDA.
Conclusion: Our findings indicate that oxidative stress, impairment of the antioxidant system and abnormal lipid metabolism may play a role in the pathogenesis and progression of ESRD and its related complications. These data suggest that patients with ESRD are more susceptible to vascular diseases.
Introduction
End-stage renal disease (ESRD) is associated with several disorders including atherosclerosis, arthritis, dyslipidemia, metabolic syndrome, and cardiovascular disease (CVD). CVD has been reported as one of the main causes of death in patients with ESRD.Citation1 A multicenter study demonstrated that the patients with ESRD have 10–20-fold higher chances to develop CVD compared with the general population and this evidence cannot be solely due to the “traditional” risk factors.Citation2 Therefore, early detection of susceptibility of patients to ESRD and to CVD is of significant value. Evidences indicate that oxidative stress is one of the important factors involved in the development of CVD and atherosclerosis in patients undergoing maintenance hemodialysis.
It has been suggested that deficiency and alterations in the antioxidant mechanism and increased production of reactive oxygen species, lipid oxidation index of malondialdehyde (MDA) and DNA damage are important participating agents in the initiation and progression of oxidative and atherogenic event.Citation3–5 In addition to the well-established link of oxidative stress with renal failure, the role of genetic predisposition in enhanced oxidative damageCitation6 is demonstrated. Soluble glutathione S-transferase (GST, N-acetyltransferase epoxide hydroxylase, and sulphotransferase) is also known as phase II detoxifying enzymes.Citation7,Citation8 play an essential role in protecting DNA from genotoxins damage by inhibiting the formation of DNA adducts.Citation9 Human GST genes are divided into four major subfamilies designated as GST α or A, GST μ or M, GST θ or T, and GST π or P.Citation8 GSTM1 and T1 are polymorphic in populations and the null genotype for each of these genes has a high prevalence in human populations (10–65%).Citation10,Citation11 Deletions of these genes lead to lack of enzyme activity.Citation12 The GSTP1 gene shows polymorphism within its coding region, of which the most well-known is an A–G transition at nucleotide position 1578, causing an isoleucine to valine substitution at codon 105 (Ile105 Val) in exon 5. This polymorphism results in decreased enzyme activity.Citation5 This mutation is associated with high level of hydrophobic DNA adducts.Citation13 In addition, altered GST activity associated with polymorphisms is expected to affect coronary artery disease (CAD), kidney disease, and cancer risk through decreased protection against DNA damage from reactive electrophiles.Citation14
In this study, we investigated the relationship of GST genotypes with the risk of ESRD, and detected the levels of MDA, and total antioxidant capacity (TAC) in ESRD patients from west population of Iran.
Patients and methods
Subjects
The study protocol was approved by the Ethics Committee of the Kermanshah University of Medical Sciences and was in accordance with the principles of the Declaration of Helsinki II. All subjects provided written informed consent. The subjects were 136 hemodialysis patients with ESRD (mean age 58.1 ± 13.3 years; 89 male and 47 female) that were going under the dialysis for at least three times a week. They were selected from the Nephrology Unit of Imam Reza hospital of the Kermanshah University of Medical Sciences. The control group consisted of 137 individuals (mean age 55.7 ± 7.3 years; 87 male and 50 female) without any obvious renal complications as determined by the level of urea and creatinine in their serum.Citation15 Controls and patients were sex and age matched.
Blood collection
A total of 5 mL of venous blood sample were collected from patients (pre-dialysis) and control subjects. Three milliliter of the blood samples were collected in EDTA vials for genomic DNA extraction and 2 mL blood without EDTA was used for biochemical analysis.
DNA extraction
DNA was extracted from blood by phenol-chloroform extraction method.Citation16 The purity and concentration of extracted DNA was measured by Nanodrop spectrophotometer (Thermo 2000C model). The forward and reverse primers used for multiplex PCR and PCR conditions for determination of GSTM1, GSTT1, and GSTP1 genotypes are listed in . β-globin gene was co-amplified and used as an internal control for multiplex PCR. Multiplex PCR was carried out using 20 pmol of each primer,Citation17 200 μM dNTPs, 1.5 mM MgCl2, 1U Taq polymerase enzyme in a 10x PCR buffer, and 300–500 ng genomic DNA in total volume of 25 μL. After the DNA was denatured at 95 °C for 8 min, the reaction mixture was subjected to 35 cycles of 94 °C for 1.0 min, 50 °C for 1.0 min, and 72 °C for 1.5 min with a final extension time of 10 min at 72 °C. PCR products were electrophoresed on a 2% agarose gel. The presence of GSST1 (a 480 bp fragment) and GSTM1 (a 230 bp fragment) was confirmed in the presence of amplified fragment of β-globin gene (268 bp). In the presence of GSTM1-null or GSTT1-null genotype, no specific PCR product of GSTM1 and GSTT1 was observed ().
Figure 1. Agarose gel electrophoresis (2%) pattern of GSTM1 and GSTT1 multiplex PCR. From left to right lane 1 shows 100 base pairs DNA molecular weight marker; GSTT1 yields a product of 480 bp, whereas the amplification product of GSTM1 is 230 bp and β-globin gene (268 bp) as an internal control. Lanes 2 GSTT1/GSTM1 genotypes, lanes 3 GSTT1/GSTM1null genotypes, lanes 4 and 7 GSTT1null/GSTM1 genotypes, lanes 5, and 6 GSTT1null/GSTM1null genotypes.
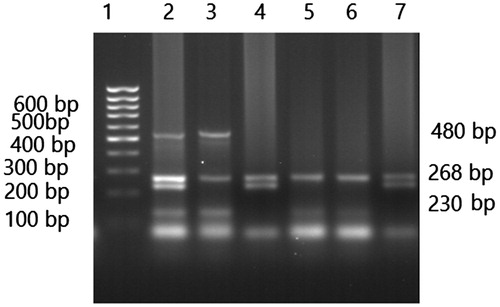
Table 1. Primers and PCR conditions for all the GSTs variants.
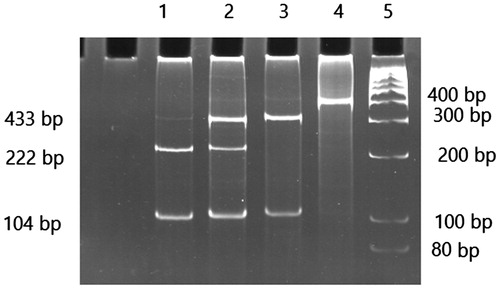
Genotyping of the single-nucleotide polymorphism of the GSTP1was performed by PCR-RFLP. The GSTP1 allele was detected by treating their corresponding PCR products with 1 U of ALW26I restriction enzyme for at least 16 h in 37 °C. The digested fragments were electrophoresed on a 12% polyacrylamide gel. The genotypes of GSTP1 were determined as follows: The PCR product (433 bp) corresponding to wild type of GSTP1 allele was cleared by ALW26 enzyme and generating 329 and 104 bp, while the PCR products containing of the homozygote genotype generating 222 and 104 bp DNA fragments and heterozygotes of GSTP1 were cleared by the enzyme producing 329, 222 and 104 bp fragments ().Citation17
Measurement of serum levels of MDA and TAC
Plasma MDA was measured by an Agilent Technologies 1200 Series high-performance liquid chromatography (HPLC) system (Agilent Corp., Germany) using a fluorescence detector. The column was EC 250/4.6 Nucleodur 100–5 C18ec (Macherey-Nagel, Duren, Germany). Butylated hydroxytoluene (BHT), MDA, methanol, 2-thiobarbituric acid (TBA), 1,1,3,3-tetraethoxypropane (TEP), were analytical grade and purchased from Sigma Chemical Co. (St Louis, MO), all other reagents were products of Merck (Darmstadt, Germany).
For MDA analysis, a 50-μL sample [plasma or TEP standard (stock standard solution containing 5 μM TEP in 40% ethanol solution] was spiked with 50 μL BHT (0.05%V/V BHT in ethanol), 400 μL H3PO4 solution, and 100 μL TBA (TBA 42 mM in 0.44 M phosphoric acid) solution in a 2-mL Eppendorf tube. Sample tubes were capped tightly, vortex mixed, then incubated for 1 h in a 100 °C water bath. Following heat derivatization, samples were cooled on an ice for 10 min, with 250 μL n-butanol subsequently added to each vial for extraction of the MDA–TBA complex. Tubes were vortex mixed 5 min then centrifuged 3 min at 14,000 g to separate the two phases. Aliquots of 100 μL were removed from the n-butanol layer of each sample and placed in HPLC vials for analysis without evaporation. Serum MDA was determined by injection of 20 μL of above solution onto HPLC reverse phase with a mobile phase comprised methanol-buffer (40/60, V/V) [phosphate buffer (KH2PO4 50 mM adjust pH = 6.7 with KOH) pumped at 1 mL/min. MDA was detected by its native fluorescence at 553 nm, excitation 515 nm. The concentration and identity of the eluted MDA was confirmed by comparison to a commercial standard and quantified by peak area using Agilent Technologies 1200 Series software.Citation18 All analysis was conducted in duplicate and data was displayed as the mean ± the standard error of the mean of duplicate treatments.
The serum levels of TAC were measured using commercially available kits (Randox Laboratories Ltd., Crumlin, Antrim, N. Ireland, Cat. no. NX2332).
Statistical analysis
Allelic frequencies were calculated by the gene counting method. The χ2 test was used to verify the agreement of the observed genotype frequencies with those expected according to the Hardy–Weinberg equilibrium. The genotypes and allele frequencies of GST in patients with ESRD were compared with the control group using the χ2 test. Odds ratios (OR) were calculated as estimates of relative risk for disease and 95% confidence intervals obtained by SPSS logistic regression (SPSS Inc., Chicago, IL). A two-tailed Student’s t-test, analysis of variance and nonparametric independent sample Mann–Whitney analysis were used to compare quantitative data. Statistical significance was assumed at p < 0.05.
Results
Characteristics of ESRD patients and control subjects are shown in . There was no significant difference between the mean of age and sex of the two groups. The patients with ESRD had significantly higher plasma MDA concentration (2.04 ± 0.4 vs. 1.1 ± 0.33 μM, p < 0.001) and serum level of TAC (8.9 ± 2.4 vs. 6.4 ± 2.3 mM, p < 0.001) compared to control group.
Table 2. Characteristics and distribution of risk factors in patients with ESRD and control subjects in a population from western Iran.
GST M1and GST T1 genotype
Frequencies of GST M1&T1 normal and null genotypes are described in detail in . The observed GST M1, T1, and P genotypes distribution in patients with ESRD and healthy individuals was the same as the predicted genotype from the Hardy–Weinberg equilibrium. The frequencies of GST normal (T1+-M1+) and GSTT1-null (T1−-M1+) genotypes were higher in control subjects than patients, while the frequencies of GSTM1-null (T1+-M−) and GST-null (T1−-M1−) genotypes were significantly higher in patients with ESRD compared to those in controls (p < 0.001, df = 3, χ2 = 22.6). Odds ratio (OR) of GST T1 and GST M1 genotypes in individuals with either GSTM1-null or GSTT1-null genotype indicated that these genotypes increased the risk of ESRD by 2.61 times, and those genotypes with one of GSTM1-null or GSTT1-null or GST-null (T1−-M1−) genotypes, increased 3.3 times risk of ESRD (). The presence of GSTM1-null genotype increased the risk of ESRD by 3.04 fold.
Table 3. Distribution of GST M1 & T1 genotypes in patients with ESRD and control subjects.
Table 4. Odds ratios of GST M1 & T1 genotypes in patients with ESRD after adjustment for sex and age.
As shown in , patients with GSTM1-null, GSTT1-null, or GST-null genotypes had significantly higher plasma concentrations of MDA and TAC compared to controls with the corresponding genotypes.
Table 5. The relationship between GST T1 & M1 genotypes with TAC and MDA between ESRD patients and control subjects.
GST P1 genotype and alleles
The overall distribution of GST P1 genotype and alleles (as calculated using the actual allele number) in ESRD patients were similar to those in control group. GST P1 genotype and alleles did not significantly increase the risk of ESRD ().
Table 6. The distribution and odds ratios of GST P1 genotypes and alleles in patients with ESRD after adjustment for sex and age.
Discussion
Chronic kidney disease (CKD) is increasingly recognized as a major public health problem in the world. ESRD is a progressive and irreversible deterioration in renal function associated with high levels of free radicals.Citation4,Citation19 Alterations and modifications in plasma lipid concentration, the antioxidant defiance mechanism, a significant increase in the levels of serum biomarkers for lipid peroxidation, such as MDA, and lower levels of non-enzymatic antioxidant systems have been observed in patients with ESRD. These results suggest that there is a relationship between ESRD and oxidative damage.
GSTs isoforms are responsible for cell defense against electrophile agents and unsaturated aldehyde atherogens.Citation20 These enzymes catalyze the conjugation of glutathione to a wide range of electrophiles and play a protective role against oxidative stress.Citation21 This study is the first report that has evaluated the role of GST polymorphism in the development of ESRD in Iranian population. The results revealed that the null genotypes of GSTM1− and GSTT1− were associated with higher risk of chronic renal failure. The GSTM1-null genotype had the most pronounced effect, it increased the risk of ESRD by 3.04 fold while, GSTT1-null and GST null GST (T1−-M1−) genotypes increased the risk of ESRD by 1.41 and by 1.8 times, respectively. The combination of the three genotypes (GST null + GSTM1-null + GSTM1-null) increased the risk of ESRD by 3.3 times. In addition, as shown in , patients with ESRD who carried GSTM1-null or a combination of GSTT1-null and GST null genotypes had significantly higher-plasma MDA level than the control group carrying the corresponding GST genotypes. These results support the hypothesis that the GSTM-1 null and GST null (T1−-M1−) genotypes are associated with increased risk of ESRD development. This is consistent with several studies performed in populations from Belgrade, Serbia,Citation6 EgyptCitation3, and Indian.Citation22–24 However, such association was not found in TaiwaneseCitation25 or Asian Indians ESRD patients.Citation26
Human cytosolic GSTs have been well characterized and are known to be polymorphic, with variable frequency in different ethnic groups. The percentage of individuals who do not express the GSTM1 and GSTT1 enzymes due to homozygous gene deletion is higher in Caucasians and in Asians than in Africans. About 60% of Asians, 40% of Africans, and 20% of Caucasians do not express the GSTM1 and GSTT1 enzymes.Citation22 These data suggest the existence of ethnic-specific GST genetic susceptibility to ESRD development.
The role of low-activity GST-P1 polymorphism has been addressed in only two studiesCitation22,Citation26 that demonstrated an association between this polymorphism with increased risk of ESRD. Our result, however, did not show significant association between GST P1 polymorphism and ESRD.
Among GST genotypes, individuals with GST null (T1−-M1−) genotype had maximum MDA levels when compared to the control group, indicating a high level of lipid peroxidation in these subjects. Recently, we have reported that GSTT1-null and GSTM1-null genotypes are involved in the pathogenesis of CAD in west of Iran and patients with SLE, psoriasis, or CVD have significantly high concentration of MDA compared to control individuals.Citation17,Citation18,Citation27,Citation28 These data together suggest a link between MDA level and high risk of CVD development in patients with ESRD.Citation29,Citation30
In this study, the frequency of GST normal in control group was 32.8% and the frequency of GST mutant (GSTM1-null + GSTT1-null + GST-null) was 67.2%, whereas in ESRD group, they were 12.8 and 87.2%, respectively. Our data suggests that inactive forms of enzymes do not protect the individuals from oxidative damage that can lead to the development of ESRD.Citation31
Conclusion
Our results indicated that the GST-null allele (GSTT1-null/GSTM1-null) is a risk factor for ESRD. Carriers of this allele have high levels of MDA. These results indicate that oxidative stress, impairment of the antioxidant system and abnormal lipid metabolism may play a role in the pathogenesis and progression of ESRD and its related complications. Our data also suggest that patients with ESRD are more susceptible to vascular diseases. However, due to the heterogeneous picture of ESRD and the influence of a subset of risk factors in the development of the disease further studies are needed to shed light on contribution of GST-null allele of GST polymorphism and their enzymatic activity in the development of ESRD in different ethnicities.
Disclosure statement
We declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work was supported by the Kermanshah University of Medical Sciences, Kermanshah, Iran [grant No. 90064].
References
- Zoccali C, Kramer A, Jager KJ. Chronic kidney disease and end-stage renal disease—a review produced to contribute to the report ‘the status of health in the European Union: Towards a healthier Europe’. NDT Plus. 2010;3:210–224.
- Lacquaniti A, Boliqnano D, Donato V, Bono C, Fazio MR, Buemi M. Alterations of lipid metabolism in chronic nephropathies: Mechanisms, diagnosis and treatment. Kidney Blood Press Res. 2010;33:100–110.
- Farouk H, Kandil D, Kamel S, Elghoroury EA, Elshamaa MF, Sabry S. Effect of GSTM1 and GSTT1 deletions in the development of oxidative stress in children with chronic kidney disease. J Clin Basic Cardiol. 2013;16:1–5.
- Libetta C, Sepe V, Esposito P, Galli F, Dal Canton A. Oxidative stress and inflammation: Implications in uremia and hemodialysis. Clin Biochem. 2011;44: 1189–1198.
- Lin YS, Hung SC, Wei YH, Tarng DC. GST M1 polymorphism associates with DNA oxidative damage and mortality among hemodialysis patients. J Am Soc Nephrol. 2009;20:405–415.
- Suvakov S, Damjanovic T, Stefanovic A. Glutathione S-transferase A1, M1, P1 and T1 null or low-activity genotypes are associated with enhanced oxidative damage among hemodialysis patients. Nephrol Dial Transplant. 2013;28:202–212.
- Buzio L, De Palma G, Mazzoni P, et al. Glutathione S-transferases M1-1 and T1-1 as risk modifiers for renal cell cancer associated with occupational exposure to chemicals. Occup EnvironMed. 2003;60:789–793.
- Mannervick B, Awasthi YC, Board PG et al. Nomenclature for human glutathione transferases. Biochem J. 1995;282:305–306.
- Vos RM, Van Bladeren PJ. Glutathione S-transferases in relation to their role in the biotransformation of xenobiotics. Chem Biol Interact. 1990;75:241–265.
- Katoh T, Naqata N, Kuroda Y, et al. Glutathione S-transferase M1 (GSTM1) and T1 (GSTT1) genetic polymorphism and susceptibility to gastric and colorectal adenocarcinoma. Carcinogenesis. 1996;17:1855–1859.
- Kumar M, Agarwal SK, Goel SK. Lung cancer risk in north Indian population: Role of genetic polymorphisms and smoking. Mol Cell Biochem. 2009;322:73–79.
- Yalin S, Hatungil R, Tamer L, et al. Glutathione S-transferase gene polymorphisms in Turkish patients with diabetes mellitus. Cell Biochem Funct. 2007;25:509–513.
- Sweeney C, Farrow DC, Schwartz SM, Eaton DL, Checkoway H, Vaughan TL. Glutathione S-transferase M1, T1, and P1 polymorphisms as risk factors for renal cell carcinoma: A casecontrol study. Cancer Epidemiol Biomarkers Prev. 2000;9:449–454.
- De flora S, Izzotti A, Walsh D, Degan P, Petrilli GL, Lewtas J. Molecular epidemiology of atherosclerosis. FASEB J. 1997;11:1021–1031.
- Foundation NK. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266.
- Kharrazi H, Vaisi-Raygani A, Rahimi Z, Tavilani H, Aminian M, Pourmotabbed T. Association between enzymatic and non-enzymatic antioxidant defense mechanism with apolipoprotein E genotypes in Alzheimer disease. Clin Biochem. 2008;41:932–936.
- Nomani H, Mozafari H, Ghobadloo SM, Rahimi Z, Raygani AV, Rahimi MA. The association between GSTT1, M1, and P1 polymorphisms with coronary artery disease in Western Iran. Mol Cell Biochem. 2011;354:181–187.
- Bahrehmand F, Vaisi-Raygani A, Rahimi Z, et al. Synergistic effects of BuChE non-UU phenotype and paraoxonase (PON1) 55 M allele on the risk of systemic lupus erythematosus: Influence on lipid and lipoprotein metabolism and oxidative stress, preliminary report. Lupus. 2014;23:263–272.
- Kao MP, Ang DS, Pall A, Struthers AD. Oxidative stress in renal dysfunction: Mechanisms, clinical sequelae and therapeutic options. J Hum Hypertens. 2010;24:1–8.
- He N, Singhal SS, Awasthi S, Zhao T, Boor PJ. Role of glutathione S-transferase 8-8 in allylamine resistance of vascular smooth muscle cells in vitro. Toxicol Appl Pharmacol. 1999;158:177–185.
- Ketterer B. Glutathione S-transferases and prevention of cellular free radical damage. Free Radic Res. 1998;28:647–658.
- Agrawal S, Tripathi G, Khan F, Sharma R, Baburaj VP. Relationship between GSTs gene polymorphism and susceptibility to end stage renal disease among North Indians. Ren Fail. 2007;29:947–953.
- Datta SK, Kumar V, Pathak R, et al. Association of glutathione Stransferase M1 and T1 gene polymorphism with oxidative stress in diabetic and nondiabetic chronic kidney disease. Ren Fail. 2010;32:1189–1195.
- Datta SK, Kumar V, Ahmed RS, Tripathi AK, Kalra OP, Banerjee BD. Effect of GSTM1 and GSTT1 double deletions in the development of oxidative stress in diabetic nephropathy patients. Indian J Biochem Biophys. 2010;47:100–103.
- Yang Y, Kao MT, Chang CC, et al. Glutathione S-transferase T1 deletion is a risk factor for developing end-stage renal disease in diabetic patients. Int J Mol Med. 2004;14:855–859.
- Tiwari AK, Prasad P, B KT, et al. Oxidative stress pathway genes and chronic renal insufficiency in Asian Indians with Type 2 diabetes. J Diabetes Complications. 2009;23:102–111.
- Asefi M, Vaisi-Raygani A, Bahrehmand F, et al. Paraoxonase 1 (PON1) 55 polymorphism, lipid profiles and psoriasis. Br J Dermatol. 2012;167:1279–1286.
- Rahimi Z, Ahmadi R, Vaisi-Raygani A, Rahimi Z, Bahrehmand F, Parsian A. Butyrylcholinesterase (BChE) activity is associated with the risk of preeclampsia: Influence on lipid and lipoprotein metabolism and oxidative stress. J Matern Fetal Neonatal Med. 2013;26:1590–1594.
- Osorio A, Ortega E, de Haro T, Torres JM, Sanchez P, Ruiz-Reguena E. Lipid profiles and oxidative stress parameters in male and female hemodialysis patients. Mol Cell Biochem. 2011;353:59–63.
- Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: Effects on the cardiovascular system. Circulation. 2007;116:85–97.
- Walker LS, Sansom DM. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev Immuno. 2011;11:852–863.